| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 20:11:33 -0600 |
|---|
| Update Date | 2015-12-09 14:11:53 -0700 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
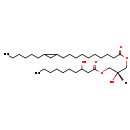
| Name: | DG(19:0cycv8c/10:0(3-OH)/0:0) |
|---|
| Description | DG(19:0cycv8c/10:0(3-OH)/0:0) belongs to the family of Diacylglycerols. These are glycerolipids lipids containing a common glycerol backbone to which at least one fatty acyl group is esterified. DG(19:0cycv8c/10:0(3-OH)/0:0) is also a substrate of diacylglycerol kinase. It is involved in the phospholipid metabolic pathway. |
|---|
| Structure | |
|---|
| Synonyms: | Not Available |
|---|
| Chemical Formula: | C32H60O6 |
|---|
| Weight: | Average: 540.826
Monoisotopic: 540.438989652 |
|---|
| InChI Key: | OGCBVSAQVVEYQF-IYCLCUOYSA-N |
|---|
| InChI: | InChI=1S/C32H60O6/c1-3-5-7-12-17-21-29(33)24-32(36)38-26-30(34)25-37-31(35)22-18-14-11-9-10-13-16-20-28-23-27(28)19-15-8-6-4-2/h27-30,33-34H,3-26H2,1-2H3/t27?,28?,29?,30-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2S)-3-{[10-(2-hexylcyclopropyl)decanoyl]oxy}-2-hydroxypropyl 3-hydroxydecanoate |
|---|
| Traditional IUPAC Name: | (2S)-3-{[10-(2-hexylcyclopropyl)decanoyl]oxy}-2-hydroxypropyl 3-hydroxydecanoate |
|---|
| SMILES: | [H][C@](O)(COC(=O)CCCCCCCCCC1CC1CCCCCC)COC(=O)CC(O)CCCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3-diacylglycerols. These are diacylglycerols containing a glycerol acylated at positions 1 and 3. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Diradylglycerols |
|---|
| Direct Parent | 1,3-diacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3-acyl-sn-glycerol
- Fatty alcohol
- Beta-hydroxy acid
- Fatty acid ester
- Dicarboxylic acid or derivatives
- Fatty acyl
- Hydroxy acid
- Carboxylic acid ester
- Secondary alcohol
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organic oxygen compound
- Carbonyl group
- Organic oxide
- Alcohol
- Organooxygen compound
- Aliphatic homomonocyclic compound
|
|---|
| Molecular Framework | Aliphatic homomonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | phospholipid biosynthesis (CL(19:0cycv8c/10:0(3-OH)/10:0/10:0)) | PW001989 | 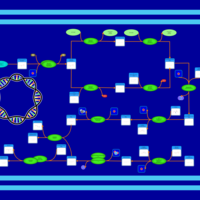 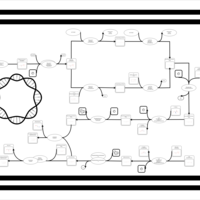 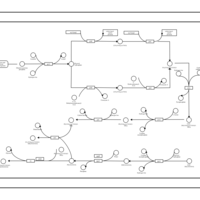 | | phospholipid biosynthesis (CL(19:0cycv8c/10:0(3-OH)/19:0cycv8c/10:0(3-OH))) | PW001991 | 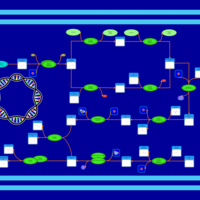 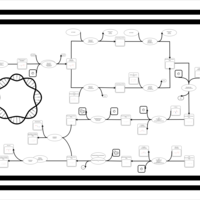 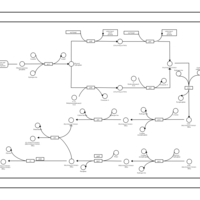 |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|