| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:50:28 -0600 |
|---|
| Update Date | 2015-09-14 16:46:47 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | dTDP-thomosamine |
|---|
| Description | dTDP-thomosamine is an intermediate in dTDP-N-acetylthomosamine biosynthesis in E.coli. It is a substrate for the enzyme dTDP-fucosamine acetyltransferase which catalyzes the reaction dTDP-thomosamine + acetyl-CoA -> dTDP-N-acetylthomosamine + coenzyme A + H+. It is also a product for enzyme dTDP-4-dehydro-6-deoxy-D-glucose transaminase which catalyzes reaction dTDP-4-dehydro-6-deoxy-α-D-glucopyranose + L-glutamate -> dTDP-thomosamine + 2-oxoglutarate (BioCyc compound: TDP-D-FUCOSAMINE). |
|---|
| Structure | |
|---|
| Synonyms: | | Value | Source |
|---|
| dTDP-4-Amino-4,6-dideoxy-alpha-D-galactose | ChEBI | | dTDP-alpha-D-Fucosamine | ChEBI | | dTDP-4-Amino-4,6-dideoxy-a-D-galactose | Generator | | dTDP-4-Amino-4,6-dideoxy-α-D-galactose | Generator | | dTDP-a-D-Fucosamine | Generator | | dTDP-Α-D-fucosamine | Generator | | dTDP-4-amino-4,6-Dideoxy-a-D-galactose(1-) | Generator | | dTDP-4-amino-4,6-Dideoxy-α-D-galactose(1-) | Generator |
|
|---|
| Chemical Formula: | C16H26N3O14P2 |
|---|
| Weight: | Average: 546.339
Monoisotopic: 546.089550105 |
|---|
| InChI Key: | UIVJXHWSIFBBCY-FQLHZTMTSA-M |
|---|
| InChI: | InChI=1S/C16H27N3O14P2/c1-6-4-19(16(24)18-14(6)23)10-3-8(20)9(31-10)5-29-34(25,26)33-35(27,28)32-15-13(22)12(21)11(17)7(2)30-15/h4,7-13,15,20-22H,3,5,17H2,1-2H3,(H,25,26)(H,27,28)(H,18,23,24)/p-1/t7-,8+,9-,10-,11+,12+,13-,15-/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 1-[(2R,4S,5R)-5-[({[({[(2R,3R,4S,5R,6R)-5-amino-3,4-dihydroxy-6-methyloxan-2-yl]oxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)methyl]-4-hydroxyoxolan-2-yl]-5-methyl-2-oxo-1,2-dihydropyrimidin-4-olate |
|---|
| Traditional IUPAC Name: | 1-[(2R,4S,5R)-5-{[({[(2R,3R,4S,5R,6R)-5-amino-3,4-dihydroxy-6-methyloxan-2-yl]oxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]methyl}-4-hydroxyoxolan-2-yl]-5-methyl-2-oxopyrimidin-4-olate |
|---|
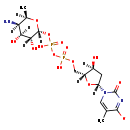
| SMILES: | [H][C@]1(O)C[C@@]([H])(O[C@]1([H])COP(O)(=O)OP(O)(=O)O[C@@]1([H])O[C@]([H])(C)[C@]([H])(N)[C@]([H])(O)[C@@]1([H])O)N1C=C(C)C([O-])=NC1=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as polyprenyl phospho carbohydrates. These are polyprenyl phosphates with a carbohydrate moiety attached to it. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Polyprenols |
|---|
| Direct Parent | Polyprenyl phospho carbohydrates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polyterpenoid
- Polyprenyl phospho carbohydrate
- Bactoprenol diphosphate
- Polyprenyl monophosphate
- Polyprenyl phosphate skeleton
- N-acyl-alpha-hexosamine
- Hexose monosaccharide
- Isoprenoid phosphate
- Monosaccharide phosphate
- Organic pyrophosphate
- Monoalkyl phosphate
- Monosaccharide
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Alkyl phosphate
- Acetamide
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Oxacycle
- Carboxylic acid derivative
- Organoheterocyclic compound
- Carbonyl group
- Organic oxide
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Primary alcohol
- Organonitrogen compound
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | Secondary Metabolites: enterobacterial common antigen biosynthesis | PW000959 |    | | Secondary Metabolites: enterobacterial common antigen biosynthesis 2 | PW002045 |    |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 68492 | | HMDB ID | Not Available | | Pubchem Compound ID | 70678920 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|