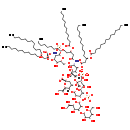| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:50:09 -0600 |
|---|
| Update Date | 2015-09-14 16:46:37 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | glucosyl-(heptosyl)2-Kdo2-lipid A-phosphate |
|---|
| Description | Glucosyl-(heptosyl)2-Kdo2-lipid A-phosphate is an intermediate in Lipid A-core biosynthesis pathway in E.coli. It is a substrate for the enzyme lipopolysaccharide core heptosyl transferase III which catalyzes the reaction glucosyl-(heptosyl)2-Kdo2-lipid A-phosphate + ADP-L-glycero-beta-D-manno-heptose -> glucosyl-(heptosyl)3-Kdo2-lipid A-phosphate + ADP + H+. It is also a product for enzyme lipopolysaccharide core heptose (I) kinase which catalyzes reaction glucosyl-(heptosyl)2-Kdo2-lipid A + ATP → glucosyl-(heptosyl)2-Kdo2-lipid A-phosphate + ADP + H+ (BioCyc compound: CPD0-933). |
|---|
| Structure | |
|---|
| Synonyms: | - Glucosyl-(heptosyl)2-kdo2-lipid a-phosphoric acid
|
|---|
| Chemical Formula: | C130H229N2O59P3 |
|---|
| Weight: | Average: 2857.143
Monoisotopic: 2855.423717595 |
|---|
| InChI Key: | GBPUUSBSBGYHGL-DOCZPCJQSA-F |
|---|
| InChI: | InChI=1S/C130H237N2O59P3/c1-7-13-19-25-31-37-38-44-50-56-62-68-98(148)175-85(66-60-54-48-42-35-29-23-17-11-5)72-100(150)180-118-102(132-96(146)71-84(65-59-53-47-41-34-28-22-16-10-4)174-97(147)67-61-55-49-43-36-30-24-18-12-6)122(172-80-93-105(153)117(179-99(149)70-83(139)64-58-52-46-40-33-27-21-15-9-3)101(123(177-93)191-194(169,170)171)131-95(145)69-82(138)63-57-51-45-39-32-26-20-14-8-2)178-94(116(118)189-192(163,164)165)81-173-129(127(159)160)74-91(186-130(128(161)162)73-86(140)103(151)112(187-130)88(142)76-134)115(114(188-129)90(144)78-136)183-126-110(158)120(121(190-193(166,167)168)113(182-126)89(143)77-135)185-125-109(157)119(108(156)111(181-125)87(141)75-133)184-124-107(155)106(154)104(152)92(79-137)176-124/h82-94,101-126,133-144,151-158H,7-81H2,1-6H3,(H,131,145)(H,132,146)(H,159,160)(H,161,162)(H2,163,164,165)(H2,166,167,168)(H2,169,170,171)/p-8/t82-,83-,84-,85-,86-,87+,88-,89+,90-,91-,92-,93-,94-,101-,102-,103-,104-,105-,106+,107-,108-,109+,110+,111-,112-,113-,114-,115-,116-,117-,118-,119+,120-,121-,122-,123-,124?,125-,126-,129-,130-/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2R,4R,5R,6R)-2-{[(2R,4R,5R,6R)-2-carboxylato-6-[(1R)-1,2-dihydroxyethyl]-5-{[(2S,3S,4R,5R,6R)-6-[(1S)-1,2-dihydroxyethyl]-4-{[(2R,3S,4S,5R,6R)-6-[(1S)-1,2-dihydroxyethyl]-3,5-dihydroxy-4-{[(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-3-hydroxy-5-(phosphonatooxy)oxan-2-yl]oxy}-2-{[(2R,3S,4R,5R,6R)-5-[(3R)-3-(dodecanoyloxy)tetradecanamido]-6-{[(2R,3S,4R,5R,6R)-3-hydroxy-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonatooxy)oxan-2-yl]methoxy}-3-(phosphonatooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxan-4-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxyoxane-2-carboxylate |
|---|
| Traditional IUPAC Name: | (2R,4R,5R,6R)-2-{[(2R,4R,5R,6R)-2-carboxylato-6-[(1R)-1,2-dihydroxyethyl]-5-{[(2S,3S,4R,5R,6R)-6-[(1S)-1,2-dihydroxyethyl]-4-{[(2R,3S,4S,5R,6R)-6-[(1S)-1,2-dihydroxyethyl]-3,5-dihydroxy-4-{[(3R,4S,5S,6R)-3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}oxan-2-yl]oxy}-3-hydroxy-5-(phosphonatooxy)oxan-2-yl]oxy}-2-{[(2R,3S,4R,5R,6R)-5-[(3R)-3-(dodecanoyloxy)tetradecanamido]-6-{[(2R,3S,4R,5R,6R)-3-hydroxy-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonatooxy)oxan-2-yl]methoxy}-3-(phosphonatooxy)-4-{[(3R)-3-(tetradecanoyloxy)tetradecanoyl]oxy}oxan-2-yl]methoxy}oxan-4-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxyoxane-2-carboxylate |
|---|
| SMILES: | [H][C@@]1(O[C@@](C[C@@H](O)[C@H]1O)(O[C@@H]1C[C@@](OC[C@H]2O[C@@H](OC[C@H]3O[C@H](OP([O-])([O-])=O)[C@H](NC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H](OC(=O)C[C@H](O)CCCCCCCCCCC)[C@@H]3O)[C@H](NC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)[C@@H](OC(=O)C[C@@H](CCCCCCCCCCC)OC(=O)CCCCCCCCCCCCC)[C@@H]2OP([O-])([O-])=O)(O[C@]([H])([C@H](O)CO)[C@@H]1O[C@H]1O[C@H]([C@@H](O)CO)[C@@H](OP([O-])([O-])=O)[C@H](O[C@H]2O[C@H]([C@@H](O)CO)[C@@H](O)[C@H](OC3O[C@H](CO)[C@@H](O)[C@H](O)[C@H]3O)[C@@H]2O)[C@@H]1O)C([O-])=O)C([O-])=O)[C@H](O)CO |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Acylaminosugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide phosphate
- Oligosaccharide
- Hexacarboxylic acid or derivatives
- Acylaminosugar
- Saccharolipid
- Hexose phosphate
- N-acyl-alpha-hexosamine
- Fatty acyl glycoside
- C-glucuronide
- Alkyl glycoside
- O-glycosyl compound
- C-glycosyl compound
- Glycosyl compound
- Fatty acid ester
- Ketal
- Beta-hydroxy acid
- Pyran
- Fatty acyl
- Phosphoric acid ester
- Oxane
- Hydroxy acid
- Organic phosphoric acid derivative
- N-acyl-amine
- Fatty amide
- Alkyl phosphate
- Carboxamide group
- Carboxylic acid ester
- Carboxylic acid salt
- Secondary carboxylic acid amide
- Secondary alcohol
- Organoheterocyclic compound
- Carboxylic acid
- Carboxylic acid derivative
- Acetal
- Oxacycle
- Polyol
- Alcohol
- Organic nitrogen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Primary alcohol
- Organonitrogen compound
- Organic anion
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -8 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | Lipopolysaccharide biosynthesis | PW000831 | 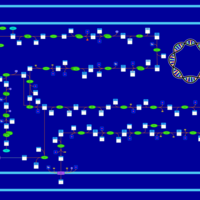 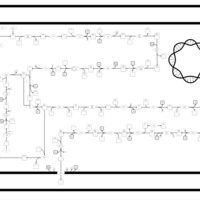 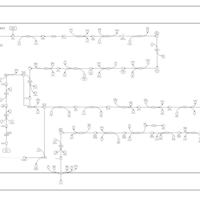 | | lipopolysaccharide biosynthesis II | PW001905 |  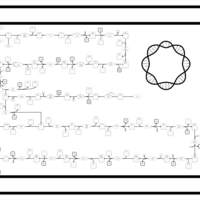  | | lipopolysaccharide biosynthesis III | PW002059 | 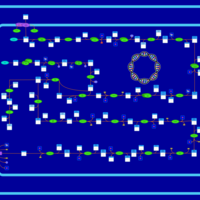 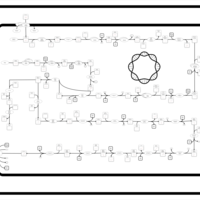 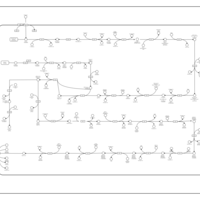 |
|
|---|
| KEGG Pathways: | - Lipopolysaccharide biosynthesis ec00540
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | Not Available |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 51351791 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|