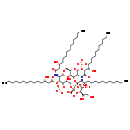| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:50:08 -0600 |
|---|
| Update Date | 2016-09-13 16:35:44 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | alpha-Kdo-(2→6)-lipid IVA |
|---|
| Description | alpha-Kdo-(2→6)-lipid IVA is an intermediate in Kdo transfer to lipid IVA I pathway in E.coli. It is a substrate for the enzyme KDO transferase which catalyzes the reaction alpha-Kdo-(2→6)-lipid IVA + CMP-3-deoxy-alpha-D-manno-octulosonate -> alpha-Kdo-(2->4)-alpha-Kdo-(2->6)-lipid IVA + CMP + H+. It is also a product for enzyme 3-deoxy-D-manno-octulosonic acid transferase which catalyzes reaction CMP-3-deoxy-alpha-D-manno-octulosonate + lipid IVA -> alpha-Kdo-(2→6)-lipid IVA + CMP + H+ (BioCyc compound: KDO-LIPID-IVA). |
|---|
| Structure | |
|---|
| Synonyms: | - a-kdo-(2→6)-lipid iva
- α-kdo-(2→6)-lipid iva
|
|---|
| Chemical Formula: | |
|---|
| Weight: | Average: Not Available
Monoisotopic: Not Available |
|---|
| InChI Key: | Not Available |
|---|
| InChI: | Not Available |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2R,4R,5R,6R)-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxy-2-{[(2R,3S,4R,5R,6R)-6-{[(2R,3S,4R,5R,6R)-3-hydroxy-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonatooxy)oxan-2-yl]methoxy}-5-[(3R)-3-hydroxytetradecanamido]-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-3-(phosphonatooxy)oxan-2-yl]methoxy}oxane-2-carboxylate |
|---|
| Traditional IUPAC Name: | (kdo)-lipid iva(5-) |
|---|
| SMILES: | Not Available |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Acylaminosugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acylaminosugar
- Saccharolipid
- Hexose phosphate
- Disaccharide phosphate
- N-acyl-alpha-hexosamine
- C-glucuronide
- C-glycosyl compound
- Disaccharide
- Glycosyl compound
- O-glycosyl compound
- Tricarboxylic acid or derivatives
- Beta-hydroxy acid
- Ketal
- Fatty acid ester
- Fatty acyl
- Fatty amide
- Alkyl phosphate
- Hydroxy acid
- N-acyl-amine
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Pyran
- Carboxamide group
- Secondary alcohol
- Secondary carboxylic acid amide
- Carboxylic acid ester
- Carboxylic acid derivative
- Carboxylic acid
- Oxacycle
- Acetal
- Organoheterocyclic compound
- Organopnictogen compound
- Alcohol
- Organic oxide
- Organic nitrogen compound
- Primary alcohol
- Carbonyl group
- Hydrocarbon derivative
- Organonitrogen compound
- Organic anion
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -5 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | Lipopolysaccharide biosynthesis | PW000831 | 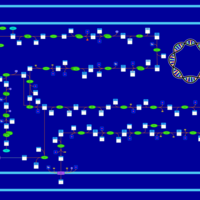 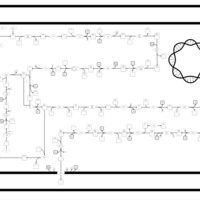 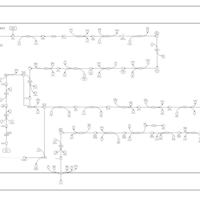 | | lipopolysaccharide biosynthesis II | PW001905 |  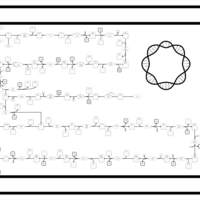  | | lipopolysaccharide biosynthesis III | PW002059 | 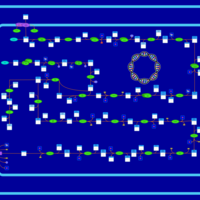 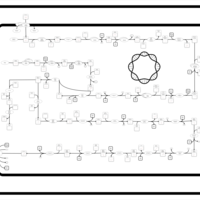 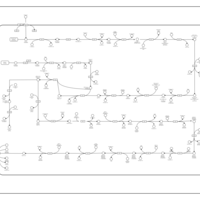 |
|
|---|
| KEGG Pathways: | - Lipopolysaccharide biosynthesis ec00540
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | Not Available |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 60364 | | HMDB ID | Not Available | | Pubchem Compound ID | 25246208 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|