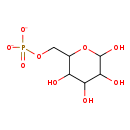| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:50:04 -0600 |
|---|
| Update Date | 2015-09-16 14:50:11 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | α-D-glucose 6-phosphate |
|---|
| Description | Glucose 6 phosphate (alpha-D-glucose 6 phosphate or G6P) is the alpha-anomer of glucose-6-phosphate. There are two anomers of glucose 6 phosphate, the alpha anomer and the beta anomer. Glucose 6 phosphate is an ester of glucose with phosphoric acid. Glucose-6-phosphate is a phosphorylated glucose molecule on carbon 6. G6P can travel down two metabolic pathways, glycolysis and the pentose phosphate pathway. It costs the cell 1 ATP to store the 1 glucose molecule and virtually no energy to remove it from storage. It is important to note that glucose-6-phosphate is an allosteric activator of glycogen synthase. On the other hand, glycogen synthase is inhibited when it is phosphorylated by protein kinase during times of high stress. -- Wikipedia. |
|---|
| Structure | |
|---|
| Synonyms: | - α-D-glucose 6-phosphoric acid
|
|---|
| Chemical Formula: | C6H11O9P |
|---|
| Weight: | Average: 258.12
Monoisotopic: 258.015166092 |
|---|
| InChI Key: | NBSCHQHZLSJFNQ-UHFFFAOYSA-L |
|---|
| InChI: | InChI=1S/C6H13O9P/c7-3-2(1-14-16(11,12)13)15-6(10)5(9)4(3)8/h2-10H,1H2,(H2,11,12,13)/p-2 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (3,4,5,6-tetrahydroxyoxan-2-yl)methyl phosphate |
|---|
| Traditional IUPAC Name: | (3,4,5,6-tetrahydroxyoxan-2-yl)methyl phosphate |
|---|
| SMILES: | OC1OC(COP([O-])([O-])=O)C(O)C(O)C1O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Hexose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose phosphate
- Monosaccharide phosphate
- Organic phosphoric acid derivative
- Oxane
- Alkyl phosphate
- Phosphoric acid ester
- Hemiacetal
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic anion
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | Amino sugar and nucleotide sugar metabolism II | PW000887 |    | | Amino sugar and nucleotide sugar metabolism III | PW000895 |    | | Galactose metabolism | PW000821 |    | | galactose degradation/Leloir Pathway | PW000884 |    |
|
|---|
| KEGG Pathways: | |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | HMDB0304534 | | Pubchem Compound ID | 4459709 | | Kegg ID | Not Available | | ChemSpider ID | 3658466 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|