| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:50:02 -0600 |
|---|
| Update Date | 2015-09-14 16:46:32 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | N5-methyl--tetrahydropteroyl tri-L-glutamate |
|---|
| Description | N5-methyl--tetrahydropteroyl tri-L-glutamate is an intermediate in pathways L-methionine biosynthesis I and S-adenosyl-L-methionine cycle I in E.coli. It is a substrate for enzyme cobalamin-independent homocysteine transmethylase in both pathways and a substrate for enzyme cobalamin-dependent methionine synthase in pathway L-methionine biosynthesis I (BioCyc compound: CPD-1302). |
|---|
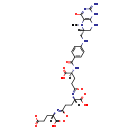
| Structure | |
|---|
| Synonyms: | - N5-Methyl--tetrahydropteroyl tri-L-glutamic acid
|
|---|
| Chemical Formula: | C30H35N9O12 |
|---|
| Weight: | Average: 713.663
Monoisotopic: 713.242711923 |
|---|
| InChI Key: | HVRNKDVLFAVCJF-VJANTYMQSA-J |
|---|
| InChI: | InChI=1S/C30H39N9O12/c1-39-16(13-33-24-23(39)26(45)38-30(31)37-24)12-32-15-4-2-14(3-5-15)25(44)36-19(29(50)51)7-10-21(41)34-17(27(46)47)6-9-20(40)35-18(28(48)49)8-11-22(42)43/h2-5,16-19,32H,6-13H2,1H3,(H,34,41)(H,35,40)(H,36,44)(H,42,43)(H,46,47)(H,48,49)(H,50,51)(H4,31,33,37,38,45)/p-4/t16-,17-,18-,19-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (4S)-4-carboxy-4-{[(4S)-4-carboxy-4-{[(4S)-4-carboxy-4-{[4-({[(6S)-2-imino-5-methyl-4-oxido-1,2,5,6,7,8-hexahydropteridin-6-yl]methyl}amino)phenyl]formamido}-1-oxidobutylidene]amino}-1-oxidobutylidene]amino}butanoate |
|---|
| Traditional IUPAC Name: | (4S)-4-carboxy-4-{[(4S)-4-carboxy-4-{[(4S)-4-carboxy-4-{[4-({[(6S)-2-imino-5-methyl-4-oxido-1,6,7,8-tetrahydropteridin-6-yl]methyl}amino)phenyl]formamido}-1-oxidobutylidene]amino}-1-oxidobutylidene]amino}butanoate |
|---|
| SMILES: | [H][C@@](CCC([O-])=N[C@@]([H])(CCC([O-])=N[C@@]([H])(CCC([O-])=O)C(O)=O)C(O)=O)(NC(=O)C1=CC=C(NC[C@@]2([H])CNC3=C(N2C)C([O-])=NC(=N)N3)C=C1)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydrofolic acids and derivatives. These are heterocyclic compounds based on the 5,6,7,8-tetrahydropteroic acid skeleton conjugated with at least one L-glutamic acid unit (or a derivative thereof) . |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Tetrahydrofolic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydrofolic acid or derivatives
- Alpha-oligopeptide
- Gamma-glutamyl alpha-amino acid
- Glutamic acid or derivatives
- Glutamine or derivatives
- Tetracarboxylic acid or derivatives
- N-acyl-l-alpha-amino acid
- Hippuric acid or derivatives
- Hippuric acid
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid or derivatives
- Aminobenzamide
- Aminobenzoic acid or derivatives
- Benzoic acid or derivatives
- Benzamide
- Benzoyl
- Phenylalkylamine
- Aniline or substituted anilines
- Dialkylarylamine
- Tertiary aliphatic/aromatic amine
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Pyrimidine
- Monocyclic benzene moiety
- Benzenoid
- N-acyl-amine
- Fatty acyl
- Fatty amide
- Vinylogous amide
- Heteroaromatic compound
- Tertiary amine
- Amino acid or derivatives
- Secondary carboxylic acid amide
- Amino acid
- Carboxamide group
- Secondary amine
- Carboxylic acid derivative
- Carboxylic acid
- Azacycle
- Carbonyl group
- Hydrocarbon derivative
- Organooxygen compound
- Primary amine
- Organic oxide
- Organic nitrogen compound
- Organopnictogen compound
- Organonitrogen compound
- Amine
- Organic oxygen compound
- Organic anion
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -3 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 58207 | | HMDB ID | Not Available | | Pubchem Compound ID | 49852302 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|