| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:50:01 -0600 |
|---|
| Update Date | 2015-09-14 16:46:09 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | L-ribulose 5-phosphate |
|---|
| Description | L-Ribulose 5-phosphate is a member of the chemical class known as Pentoses. These are monosaccharides in which the carbohydrate moiety contains five carbon atoms. In Escherichia coli, RpiA catalyzes the interconversion of ribose-5-phosphate and ribulose-5-phosphate and is a key enzyme in the pentose phosphate pathway. (PMID 12182339) Interconversion of D-ribose-5-phosphate (R5P) and D-ribulose-5-phosphate is an important step in the pentose phosphate pathway. (PMID 18640127) A key player in LPS synthesis is the enzyme D-arabinose-5-phosphate isomerase (API), which catalyzes the reversible isomerization of D-ribulose-5-phosphate to D-arabinose-5-phosphate, a precursor of 3-deoxy-D-manno-octulosonate that is an essential residue of the LPS inner core. (PMID 20954237) Dihydroxybutanone phosphate synthase (DS) catalyzes a commitment step in riboflavin biosynthesis where ribulose 5-phosphate is converted to dihydroxybutanone phosphate and formate. (PMID 11053863) Ribose-5-phosphate isomerase A (RpiA) plays an important role in interconverting between ribose-5-phosphate (R5P) and ribulose-5-phosphate in the pentose phosphate pathway and the Calvin cycle. (PMID 19214439) |
|---|
| Structure | |
|---|
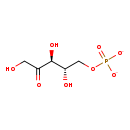
| Synonyms: | - L-Ribulose 5-phosphoric acid
|
|---|
| Chemical Formula: | C5H9O8P |
|---|
| Weight: | Average: 228.094
Monoisotopic: 228.004601408 |
|---|
| InChI Key: | FNZLKVNUWIIPSJ-CRCLSJGQSA-L |
|---|
| InChI: | InChI=1S/C5H11O8P/c6-1-3(7)5(9)4(8)2-13-14(10,11)12/h4-6,8-9H,1-2H2,(H2,10,11,12)/p-2/t4-,5+/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (3S,4S)-1,3,4-trihydroxy-5-(phosphonatooxy)pentan-2-one |
|---|
| Traditional IUPAC Name: | L-ribulose 5-phosphate(2-) |
|---|
| SMILES: | OCC(=O)[C@@H](O)[C@@H](O)COP([O-])([O-])=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Pentose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentose phosphate
- Pentose-5-phosphate
- Monosaccharide phosphate
- Acyloin
- Beta-hydroxy ketone
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Alpha-hydroxy ketone
- Ketone
- Secondary alcohol
- Polyol
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Primary alcohol
- Alcohol
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 58226 | | HMDB ID | Not Available | | Pubchem Compound ID | 21145006 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|