| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:50:00 -0600 |
|---|
| Update Date | 2015-09-08 17:50:00 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
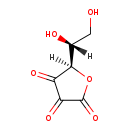
| Name: | dehydroascorbate (bicyclic form) |
|---|
| Description | |
|---|
| Structure | |
|---|
| Synonyms: | - Dehydroascorbic acid (bicyclic form)
|
|---|
| Chemical Formula: | C6H6O6 |
|---|
| Weight: | Average: 174.1082
Monoisotopic: 174.016437924 |
|---|
| InChI Key: | SBJKKFFYIZUCET-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C6H6O6/c7-1-2(8)5-3(9)4(10)6(11)12-5/h2,5,7-8H,1H2 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (5R)-5-[(1R)-1,2-dihydroxyethyl]oxolane-2,3,4-trione |
|---|
| Traditional IUPAC Name: | (5R)-5-[(1R)-1,2-dihydroxyethyl]oxolane-2,3,4-trione |
|---|
| SMILES: | OCC(O)C1OC(=O)C(=O)C1=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as gamma butyrolactones. Gamma butyrolactones are compounds containing a gamma butyrolactone moiety, which consists of an aliphatic five-member ring with four carbon atoms, one oxygen atom, and bears a ketone group on the carbon adjacent to the oxygen atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Lactones |
|---|
| Sub Class | Gamma butyrolactones |
|---|
| Direct Parent | Gamma butyrolactones |
|---|
| Alternative Parents | |
|---|
| Substituents | - 3-furanone
- Gamma butyrolactone
- Tetrahydrofuran
- 1,2-diol
- Carboxylic acid ester
- Cyclic ketone
- Secondary alcohol
- Ketone
- Carboxylic acid derivative
- Oxacycle
- Monocarboxylic acid or derivatives
- Alcohol
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Primary alcohol
- Organooxygen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9200000000-0a414c4d59046ece51c1 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fki-9831000000-c4b46024e1624e983ac3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-004i-4900000000-25a652482451303b3ca4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-1900000000-f1d23791c8a7b59576a3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4r-2900000000-5ebc67e43b07167f34cb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0abc-9000000000-27ae8f5e1795d4f732a8 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0229-0900000000-ee719ada45193fc650a0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0mbc-1900000000-d54b8e72e08e6ea1f7c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0btc-9200000000-d07ff0053ddc28ce1915 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|