| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:49:59 -0600 |
|---|
| Update Date | 2015-09-14 16:46:30 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | N2-succinylglutamate |
|---|
| Description | N2-succinylglutamate is an intermediate in L-arginine degradation II pathway in E.coli. It is a product for the enzyme succinylglutamate desuccinylase which catalyzes the reaction N2-succinyl-L-glutamate 5-semialdehyde + NAD+ + H2O -> N2-succinylglutamate + NADH + 2 H+. It is also the substrate for the enzyme succinylglutamate semialdehyde dehydrogenase which catalyzes the reaction N2-succinylglutamate + H2O -> succinate + L-glutamate (BioCyc: N2-SUCCINYLGLUTAMATE). |
|---|
| Structure | |
|---|
| Synonyms: | |
|---|
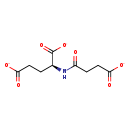
| Chemical Formula: | C9H10NO7 |
|---|
| Weight: | Average: 244.181
Monoisotopic: 244.047372406 |
|---|
| InChI Key: | JCNBNOQGFSXOML-YFKPBYRVSA-K |
|---|
| InChI: | InChI=1S/C9H13NO7/c11-6(2-4-8(14)15)10-5(9(16)17)1-3-7(12)13/h5H,1-4H2,(H,10,11)(H,12,13)(H,14,15)(H,16,17)/p-3/t5-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2S)-2-(3-carboxylatopropanamido)pentanedioate |
|---|
| Traditional IUPAC Name: | N-succinyl-L-glutamate |
|---|
| SMILES: | [O-]C(=O)CC[C@H](NC(=O)CCC([O-])=O)C([O-])=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as glutamic acid and derivatives. Glutamic acid and derivatives are compounds containing glutamic acid or a derivative thereof resulting from reaction of glutamic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Glutamic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Glutamic acid or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- N-acyl-l-alpha-amino acid
- Tricarboxylic acid or derivatives
- Fatty amide
- N-acyl-amine
- Fatty acyl
- Carboxamide group
- Secondary carboxylic acid amide
- Carboxylic acid
- Carbonyl group
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -3 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 58763 | | HMDB ID | Not Available | | Pubchem Compound ID | 25244383 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|