| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:49:59 -0600 |
|---|
| Update Date | 2016-09-13 16:35:43 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | N-carbamoyl-L-aspartate |
|---|
| Description | N-carbamoyl-L-aspartate, also known as ureidosuccinic acid, is an intermediary product in pyrimidine metabolism. It is generated by the action of dihydroorotase which catalyzes the following reaction: 4,5-Dihydroorotic acid + Water <=> Ureidosuccinic acid + H+ |
|---|
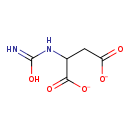
| Structure | |
|---|
| Synonyms: | - N-Carbamoyl-L-aspartic acid
|
|---|
| Chemical Formula: | |
|---|
| Weight: | Average: Not Available
Monoisotopic: Not Available |
|---|
| InChI Key: | Not Available |
|---|
| InChI: | Not Available |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 2-[(C-hydroxycarbonimidoyl)amino]butanedioate |
|---|
| Traditional IUPAC Name: | 2-(C-hydroxycarbonimidoylamino)butanedioate |
|---|
| SMILES: | Not Available |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aspartic acid and derivatives. Aspartic acid and derivatives are compounds containing an aspartic acid or a derivative thereof resulting from reaction of aspartic acid at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Aspartic acid and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aspartic acid or derivatives
- N-carbamoyl-alpha-amino acid or derivatives
- N-carbamoyl-alpha-amino acid
- Fatty acid
- Dicarboxylic acid or derivatives
- Urea
- Carbonic acid derivative
- Carboxylic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organic anion
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | Not Available |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 90158 | | HMDB ID | Not Available | | Pubchem Compound ID | 21960964 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|