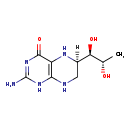
| Synonyms: | | Value | Source |
|---|
| (-)-(6R)-2-Amino-6-((1R,2S)-1,2-dihydroxypropyl)-5,6,7,8-tetrahydro-4(3H)-pteridinone | ChEBI | | (6R)-L-Erythro-5,6,7,8-tetrahydrobiopterin | ChEBI | | (6R)-L-Erythro-tetrahydrobiopterin | ChEBI | | 2-Amino-6-(1,2-dihydroxypropyl)-5,6,7,8-tetrahydoro-4(1H)-pteridinone | ChEBI | | 5,6,7,8-Tetrahydrobiopterin | ChEBI | | 6R-5,6,7,8-Tetrahydrobiopterin | ChEBI | | 6R-BH4 | ChEBI | | 6R-L-5,6,7,8-Tetrahydrobiopterin | ChEBI | | R-THBP | ChEBI | | Sapropterina | ChEBI | | Sapropterinum | ChEBI | | Tetrahydrobiopterin | ChEBI | | 5,6,7,8-erythro-Tetrahydrobiopterin | MeSH, HMDB | | 5,6,7,8-tetrahydro-L-Erythrobiopterin | MeSH, HMDB | | 5,6,7,8-Tetrahydrobiopterin, (S-(r*,s*))-isomer | MeSH, HMDB | | 5,6,7,8-Tetrahydrodictyopterin | MeSH, HMDB | | 6R-L-erythro-5,6,7,8-Tetrahydrobiopterin | MeSH, HMDB | | BPH4 | MeSH, HMDB | | D-threo-Tetrahydrobiopterin | MeSH, HMDB | | THBP | MeSH, HMDB | | Kuvan | MeSH, HMDB | | Phenylalanine hydroxylase cofactor | MeSH, HMDB | | Sapropterin dihydrochloride | MeSH, HMDB | | tetrahydro-6-Biopterin | MeSH, HMDB | | 2',4',5'-Trihydroxybutyrophenone | MeSH | | Sapropterin | MeSH | | Trihydroxybutyrophenone | MeSH | | 1-Butanone, 1-(2,4,5-trihydroxyphenyl) | MeSH | | 2,4,5-Trihydroxybutyrophenone | MeSH | | (6R)-5,6,7,8-Tetrahydro-L-biopterin | HMDB | | (6R)-5,6,7,8-Tetrahydrobiopterin | HMDB | | (6R)-Tetrahydrobiopterin | HMDB | | 2-Amino-6-(1,2-dihydroxypropyl)-5,6,7,8-tetrahydro-4(3H)-pteridinone | HMDB | | 6R-Tetrahydro-L-biopterin | HMDB | | 6beta-5,6,7,8-Tetrahydro-L-biopterin | HMDB | | 6β-5,6,7,8-Tetrahydro-L-biopterin | HMDB | | L-erythro-Tetrahydrobiopterin | HMDB | | (6R)-2-Amino-6-[(1R,2S)-1,2-dihydroxypropyl]-5,6,7,8-tetrahydro-4(1H)-pteridinone | HMDB |
|
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (6 TMS) | splash10-0zfr-2921300000-63bf6ee58b9df85919f6 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0zfr-2921300000-63bf6ee58b9df85919f6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0005-9810000000-1bfd11724596b460cae9 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-014i-6945000000-07faa91218e86d3bfe0d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 4V, positive | splash10-0006-0290000000-cd4c8c11b8279075b00a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 5V, positive | splash10-0006-0390000000-35dddce9e2df2a9ac198 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 7V, positive | splash10-0006-0590000000-cda875209a034e44cbe1 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 10V, positive | splash10-00kf-0980000000-53721c484501c528e7a8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 15V, positive | splash10-014l-0930000000-533fabd35a8c5ca7830e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 17V, positive | splash10-014i-0910000000-580c038db540047891c8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 20V, positive | splash10-014i-0910000000-8d6b754dadadb6330906 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 3V, positive | splash10-0006-0090000000-f21b677255493f1f2d1f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 23V, positive | splash10-014i-0900000000-5c1b60d0d7ea4dd4dbff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 25V, positive | splash10-014i-1900000000-6f22457ab99b14bb653e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 27V, positive | splash10-014i-1900000000-cd0410f4da61716c7f9e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - QTOF 30V, positive | splash10-014i-2900000000-d4f18a979b6d07e83a63 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 16V, positive | splash10-0002-0900000000-87174b78444828c3b52d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 16V, positive | splash10-0a4i-0900000000-dc272a476d306f26c722 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 16V, positive | splash10-014i-0900000000-ae19513d01a0046ed2e8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 16V, positive | splash10-004i-0900000000-d4a52489d35bde573bd0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 16V, positive | splash10-0f79-0900000000-af02b0d9eb8f6577afb8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - n/a 16V, positive | splash10-014i-0900000000-c2259602e8f1fd522fd0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Orbitrap 4V, positive | splash10-0006-0090000000-c88ed5ea9e11715bc275 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-006x-0090000000-e6b01d1139ccb3c6338d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0gi3-0980000000-8963ef41f1138b05a813 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-00xr-1900000000-bcfa359703563c696c0f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0390000000-4810efa20f31adea3824 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-006y-1930000000-5b32feeb643e238bb159 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-a7dbebeb1df4ea110948 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|