| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:49:15 -0600 |
|---|
| Update Date | 2015-12-09 15:39:54 -0700 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
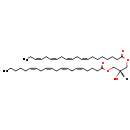
| Name: | DG(20:4(8Z,11Z,14Z,17Z)/20:4(5Z,8Z,11Z,14Z)/0:0) |
|---|
| Description | DG(20:4(8Z,11Z,14Z,17Z)/20:4(5Z,8Z,11Z,14Z)/0:0) belongs to the family of Diacylglycerols. These are glycerolipids lipids containing a common glycerol backbone to which at least one fatty acyl group is esterified. DG(20:4(8Z,11Z,14Z,17Z)/20:4(5Z,8Z,11Z,14Z)/0:0) is also a substrate of diacylglycerol kinase. It is involved in the phospholipid metabolic pathway. |
|---|
| Structure | |
|---|
| Synonyms: | | Value | Source |
|---|
| Diacylglycerol(20:4W6/0:0/20:4W3) | HMDB | | DAG(20:4/0:0/20:4) | HMDB | | Diacylglycerol(20:4/0:0/20:4) | HMDB | | Diacylglycerol(40:8) | HMDB | | 1-Arachidonoyl-3-eicsoatetraenoyl-sn-glycerol | HMDB | | Diacylglycerol(20:4n6/0:0/20:4n3) | HMDB | | DG(20:4W6/0:0/20:4W3) | HMDB | | DG(20:4/0:0/20:4) | HMDB | | DAG(20:4W6/0:0/20:4W3) | HMDB | | Diacylglycerol | HMDB | | DAG(20:4N6/0:0/20:4N3) | HMDB | | DG(40:8) | HMDB | | DAG(40:8) | HMDB | | 1-(5Z,8Z,11Z,14Z-Eicosatetraenoyl)-3-(8Z,11Z,14Z,17Z-eicosapentaenoyl)-sn-glycerol | HMDB | | Diglyceride | HMDB | | DG(20:4n6/0:0/20:4n3) | Lipid Annotator | | (2S)-2-Hydroxy-3-[(5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoyloxy]propyl (8Z,11Z,14Z,17Z)-icosa-8,11,14,17-tetraenoic acid | Generator |
|
|---|
| Chemical Formula: | C43H68O5 |
|---|
| Weight: | Average: 665.012
Monoisotopic: 664.50667529 |
|---|
| InChI Key: | PRGANJXLUCBZSQ-VANPREPPSA-N |
|---|
| InChI: | InChI=1S/C43H68O5/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-31-33-35-37-42(45)47-39-41(44)40-48-43(46)38-36-34-32-30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h5,7,11-14,17-20,23-26,30,32,41,44H,3-4,6,8-10,15-16,21-22,27-29,31,33-40H2,1-2H3/b7-5-,13-11-,14-12-,19-17-,20-18-,25-23-,26-24-,32-30-/t41-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2S)-2-hydroxy-3-[(5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoyloxy]propyl (8Z,11Z,14Z,17Z)-icosa-8,11,14,17-tetraenoate |
|---|
| Traditional IUPAC Name: | (2S)-2-hydroxy-3-[(5Z,8Z,11Z,14Z)-icosa-5,8,11,14-tetraenoyloxy]propyl (8Z,11Z,14Z,17Z)-icosa-8,11,14,17-tetraenoate |
|---|
| SMILES: | [H][C@](O)(COC(=O)CCCCCC\C=C/C\C=C/C\C=C/C\C=C/CC)COC(=O)CCC\C=C/C\C=C/C\C=C/C\C=C/CCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as other hydroxyeicosapolyenoic acids. These are hydroxyeicosapolyenoic acids which do not belong to the Hydroxyeicosapentaenoic acids, the Hydroxyeicosatetraenoic acids, or the Hydroxyeicosatrienoic acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Fatty Acyls |
|---|
| Sub Class | Eicosanoids |
|---|
| Direct Parent | Other hydroxyeicosapolyenoic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hydroxyeicosapolyenoic acid
- 1,3-acyl-sn-glycerol
- Diacylglycerol
- Diradylglycerol
- Fatty acid ester
- Glycerolipid
- Dicarboxylic acid or derivatives
- Carboxylic acid ester
- Secondary alcohol
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxygen compound
- Organic oxide
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014r-1056009000-ac94e13eda9c9374db7e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-06rb-2194022000-e622a4c534fef6a9417b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-052n-1391000000-5b49430a47506e7e659e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0w2i-0069007000-652ae0d466985b0a14d3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0udr-1059000000-94b7fadc2e17fe461967 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0zg0-3096000000-3082a4f97d2fcd728dfe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-2014095000-e959f22e33924c59c969 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-01t9-1049020000-e916f7c23ea4a856299b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004j-1189020000-3929a6e6d9bc3aadb97d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0bt9-1009003000-e2462ed8c5e5071a0955 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0ly9-4149000000-94f43534d257e6631b9a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0udr-4059000000-badd8220db20427a17be | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | HMDB0056338 | | Pubchem Compound ID | 131802035 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|