| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:49:12 -0600 |
|---|
| Update Date | 2015-09-08 17:49:12 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
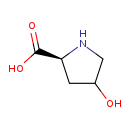
| Name: | 4-Hydroxy-L-proline |
|---|
| Description | |
|---|
| Structure | |
|---|
| Synonyms: | | Value | Source |
|---|
| (2S)-4-Hydroxypyrrolidine-2-carboxylic acid | ChEBI | | cis-4-Hydroxy-L-proline | ChEBI | | Hydroxy-L-proline | ChEBI | | L-Hydroxyproline | ChEBI | | (2S)-4-Hydroxypyrrolidine-2-carboxylate | Generator | | (4S)-4-Hydroxy-L-proline | HMDB | | allo-4-Hydroxy-L-proline | HMDB | | allo-4-Hydroxyproline | HMDB | | allo-Hydroxy-L-proline | HMDB | | cis-4-Hydroxyproline | HMDB, MeSH | | L-4-allo-Hydroxy-proline | HMDB | | L-4-Hydroxy-proline | HMDB | | L-4-Hydroxyproline | HMDB | | L-Allohydroxyproline | HMDB | | 4-Hydroxyproline | MeSH | | Oxyproline | MeSH | | cis 4 Hydroxyproline | MeSH | | Hydroxyproline | MeSH | | 4 Hydroxyproline | MeSH |
|
|---|
| Chemical Formula: | C5H9NO3 |
|---|
| Weight: | Average: 131.1299
Monoisotopic: 131.058243159 |
|---|
| InChI Key: | PMMYEEVYMWASQN-BKLSDQPFSA-N |
|---|
| InChI: | InChI=1S/C5H9NO3/c7-3-1-4(5(8)9)6-2-3/h3-4,6-7H,1-2H2,(H,8,9)/t3?,4-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2S)-4-hydroxypyrrolidine-2-carboxylic acid |
|---|
| Traditional IUPAC Name: | 4 hydroxyproline |
|---|
| SMILES: | OC1CN[C@@H](C1)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as proline and derivatives. Proline and derivatives are compounds containing proline or a derivative thereof resulting from reaction of proline at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Proline and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Proline or derivatives
- Alpha-amino acid
- L-alpha-amino acid
- Pyrrolidine carboxylic acid
- Pyrrolidine carboxylic acid or derivatives
- Pyrrolidine
- 1,2-aminoalcohol
- Amino acid
- Secondary alcohol
- Carboxylic acid
- Secondary aliphatic amine
- Monocarboxylic acid or derivatives
- Azacycle
- Secondary amine
- Organoheterocyclic compound
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic oxygen compound
- Amine
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-000x-0940000000-a668e3cb89c27d5aaaec | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-07g3-9100000000-7e4ece693ce885f2589c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0a4i-5910000000-8b634c853b803e2ce4d3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-9000000000-268874d675b6738960f8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , negative | splash10-001i-0900000000-cc0c0c49fda47ff3c20a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-001i-1900000000-091663d1fbe737185a8a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-000i-9100000000-b2aeae336cfe73f5d96e | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-014r-9000000000-cd33ebf7746baae48364 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-014i-9000000000-f7d527bb54521981a624 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-014i-9000000000-1c34470907bfbc038d07 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-000i-9000000000-2b450c503f28b7e56789 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-000i-9000000000-3885e554a98e35271b27 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-ITFT , positive | splash10-000i-9000000000-59edfed4387af678c85b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-03di-3900000000-c0b2003453a19bfc37d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-02t9-9400000000-1e365ce40f4342e10983 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9000000000-95dfbe4457c528ad98ed | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-001i-2900000000-4328dec4e0cf083ba05d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03yi-8900000000-a8cabd8deaa24da3b219 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05mo-9000000000-1216ea21a7fab26c6cf7 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|