| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:48:57 -0600 |
|---|
| Update Date | 2015-09-08 17:48:57 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | N5-Formyl-THF |
|---|
| Description | |
|---|
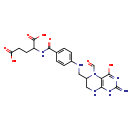
| Structure | |
|---|
| Synonyms: | | Value | Source |
|---|
| Leucovorin | Kegg | | Rescuvolin | Kegg | | 5-Formyltetrahydrofolate | Kegg | | L(-)-5-Formyl-5,6,7,8-tetrahydrofolic acid | Kegg | | 5-Formyltetrahydrofolic acid | Generator | | L(-)-5-Formyl-5,6,7,8-tetrahydrofolate | Generator | | (6R,S)-5-Formyltetrahydrofolate | HMDB | | 10-Formyl-7,8-dihydrofolate | HMDB | | 10-Formyl-7,8-dihydrofolic acid | HMDB | | 5-Formyl-5,6,7,8-tetrahydrofolate | HMDB | | 5-Formyl-5,6,7,8-tetrahydrofolic acid | HMDB | | 5-Formyltetrahydropteroylglutamate | HMDB | | 5-Formyltetrahydropteroylglutamic acid | HMDB | | Folinate | HMDB, Generator | | Folinic acid | HMDB, MeSH | | Folinic acid-SF | HMDB | | L-Leucovorin | HMDB | | L-N-[P-[[(2-amino-5-Formyl-5,6,7,8-tetrahydro-4-hydroxy-6-pteridinyl)methyl]amino]benzoyl]-glutamic acid | HMDB | | Leucal | HMDB | | Levoleucovorin | HMDB | | N5-Formyl-5,6,7,8-tetrahydrofolate | HMDB | | N5-Formyl-5,6,7,8-tetrahydrofolic acid | HMDB | | N5-Formyltetrahydrofolate | HMDB | | N5-Formyltetrahydrofolic acid | HMDB | | Welcovorin | HMDB | | Acid, folinic | MeSH, HMDB | | Folinate, calcium | MeSH, HMDB | | Leucovorin, (D)-isomer | MeSH, HMDB | | Leucovorin, (DL)-isomer | MeSH, HMDB | | Leucovorin, calcium (1:1) salt | MeSH, HMDB | | 5 Formyltetrahydrofolate | MeSH, HMDB | | Citrovorum factor | MeSH, HMDB | | Factor, citrovorum | MeSH, HMDB | | Folinic acid SF | MeSH, HMDB | | Leucovorin, calcium (1:1) salt, pentahydrate | MeSH, HMDB | | Leukovorin | MeSH, HMDB | | N(5)-Formyltetrahydrofolate | MeSH, HMDB | | Leucovorin, (R)-isomer | MeSH, HMDB | | Leucovorin, calcium (1:1) salt, (DL)-isomer | MeSH, HMDB | | Leukovorum | MeSH, HMDB | | Monosodium salt leucovorin | MeSH, HMDB | | Wellcovorin | MeSH, HMDB | | 5 Formyltetrahydropteroylglutamate | MeSH, HMDB | | Calcium folinate | MeSH, HMDB | | Calcium leucovorin | MeSH, HMDB | | Leucovorin, calcium | MeSH, HMDB | | Leucovorin, monosodium salt | MeSH, HMDB | | 5-Formlyl-5,6,7,8-tetrahydrofolate,calcium salt | Generator |
|
|---|
| Chemical Formula: | C20H23N7O7 |
|---|
| Weight: | Average: 473.4393
Monoisotopic: 473.165896125 |
|---|
| InChI Key: | VVIAGPKUTFNRDU-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C20H23N7O7/c21-20-25-16-15(18(32)26-20)27(9-28)12(8-23-16)7-22-11-3-1-10(2-4-11)17(31)24-13(19(33)34)5-6-14(29)30/h1-4,9,12-13,22H,5-8H2,(H,24,31)(H,29,30)(H,33,34)(H4,21,23,25,26,32) |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 2-[(4-{[(2-amino-5-formyl-4-oxo-3,4,5,6,7,8-hexahydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioic acid |
|---|
| Traditional IUPAC Name: | 2-[(4-{[(2-amino-5-formyl-4-oxo-3,6,7,8-tetrahydropteridin-6-yl)methyl]amino}phenyl)formamido]pentanedioic acid |
|---|
| SMILES: | NC1=NC2=C(N(C=O)C(CNC3=CC=C(C=C3)C(=O)NC(CCC(O)=O)C(O)=O)CN2)C(=O)N1 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as tetrahydrofolic acids. These are heterocyclic compounds based on the 5,6,7,8-tetrahydropteroic acid skeleton conjugated with at least one L-glutamic acid unit. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Tetrahydrofolic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tetrahydrofolic acid
- Glutamic acid or derivatives
- N-acyl-alpha amino acid or derivatives
- N-acyl-alpha-amino acid
- Hippuric acid
- Hippuric acid or derivatives
- Alpha-amino acid or derivatives
- Aminobenzamide
- Aminobenzoic acid or derivatives
- Benzamide
- Benzoic acid or derivatives
- Benzoyl
- Aniline or substituted anilines
- Phenylalkylamine
- Aminopyrimidine
- Secondary aliphatic/aromatic amine
- Pyrimidone
- Dicarboxylic acid or derivatives
- Benzenoid
- Pyrimidine
- Monocyclic benzene moiety
- Heteroaromatic compound
- Vinylogous amide
- Tertiary carboxylic acid amide
- Secondary carboxylic acid amide
- Amino acid or derivatives
- Amino acid
- Carboxamide group
- Secondary amine
- Carboxylic acid
- Carboxylic acid derivative
- Azacycle
- Organooxygen compound
- Carbonyl group
- Organic nitrogen compound
- Hydrocarbon derivative
- Amine
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Primary amine
- Organonitrogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004i-2332900000-5c40a0014d3bb486cb20 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fk9-7225198000-aa48aa22224db36842c7 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_5) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_6) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_7) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_8) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_9) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_10) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_11) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_12) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_13) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_14) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_15) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_16) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_17) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_19) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_20) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4i-0031900000-31498c227b611f3360ea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a6r-0393400000-f81606ae4f4b27aadbac | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4i-1591000000-825bc8196995ef0bc1e3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0000900000-203ae236b032df3233d7 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0umj-1131900000-5174748fd5288f6523fd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0007-9674000000-192da85bfca095ec870e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00b9-0018900000-5cbc8e2351f8b3ec2868 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0139300000-a45a2d1a5cd350ef7860 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0012-1956000000-611d4066a036a7ff475f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0001900000-20cea700a6b8ce876f5e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fb9-1304900000-85021f1e80280eb64d56 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fba-3517900000-75ddfa6e2a4565261466 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | HMDB0258132 | | Pubchem Compound ID | Not Available | | Kegg ID | C03479 | | ChemSpider ID | 140 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|