| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:48:56 -0600 |
|---|
| Update Date | 2015-09-14 16:46:05 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
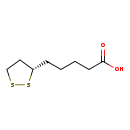
| Name: | (R)-lipoic acid |
|---|
| Description | Lipoic acid is a vitamin-like antioxidant that acts as a free-radical scavenger. Alpha-lipoic acid is also known as thioctic acid. Lipoic acid contains two thiol groups which may be either oxidized or reduced. The reduced form is known as dihydrolipoic acid (DHLA). Lipoic acid (Delta E= -0.288) is therefore capable of thiol-disulfide exchange, giving it antioxidant activity. Lipoate is a critical cofactor for aerobic metabolism, participating in the transfer of acyl or methylamine groups via the 2-Oxoacid dehydrogenase (2-OADH) or alpha-ketoglutarate dehydrogenase complex. This enzyme catalyzes the conversion of alpha-ketoglutarate to succinyl CoA. This activity results in the catabolism of the branched chain amino acids (leucine, isoleucine and valine). Lipoic acid also participates in the glycine cleavage system(GCV). The glycine cleavage system is a multi-enzyme complex that catalyzes the oxidation of glycine to form 5,10 methylene tetrahydrofolate, an important cofactor in nucleic acid synthesis. Since Lipoic acid is an essential cofactor for many enzyme complexes, it is essential for aerobic life as we know it. Lipoic acid was first postulated to be an effective antioxidant when it was found it prevented vitamin C and vitamin E deficiency. It is able to scavenge reactive oxygen species and reduce other metabolites, such as glutathione or vitamins, maintaining a healthy cellular redox state. Lipoic acid has been shown in cell culture experiments to increase cellular uptake of glucose by recruiting the glucose transporter GLUT4 to the cell membrane. (Wikipedia) |
|---|
| Structure | |
|---|
| Synonyms: | |
|---|
| Chemical Formula: | C8H14O2S2 |
|---|
| Weight: | Average: 206.326
Monoisotopic: 206.043521072 |
|---|
| InChI Key: | AGBQKNBQESQNJD-ZETCQYMHSA-N |
|---|
| InChI: | InChI=1S/C8H14O2S2/c9-8(10)4-2-1-3-7-5-6-11-12-7/h7H,1-6H2,(H,9,10)/t7-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 5-[(3S)-1,2-dithiolan-3-yl]pentanoic acid |
|---|
| Traditional IUPAC Name: | S-LA |
|---|
| SMILES: | [H][C@]1(CCCCC(O)=O)CCSS1 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as lipoic acids and derivatives. Lipoic acids and derivatives are compounds containing a lipoic acid moiety (or a derivative thereof), which consists of a pentanoic acid (or derivative) attached to the C3 carbon atom of a 1,2-dithiolane ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Dithiolanes |
|---|
| Sub Class | Lipoic acids and derivatives |
|---|
| Direct Parent | Lipoic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Lipoic_acid_derivative
- Medium-chain fatty acid
- Heterocyclic fatty acid
- Thia fatty acid
- Fatty acyl
- Fatty acid
- 1,2-dithiolane
- Organic disulfide
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organooxygen compound
- Organic oxide
- Organic oxygen compound
- Carbonyl group
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0920000000-cf913d1d1afc04e5450c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0bti-5910000000-294c510229247ef63f20 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0bvi-9600000000-f8f54eb79c5d4d5bef48 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0920000000-5a823a30dd1fb434e5b1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0kmu-1910000000-6f491a68afd82922e71b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9200000000-330e4766f82ed571497b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0a4r-0970000000-0ae5b11a032d11c1ba6d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-1900000000-decf255617c1bbd69bfb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-6900000000-eb58a3d8135ba85d4283 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-0190000000-014d2ea36cfae0726a9e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4i-5950000000-4e2dd5ccdcd5b0a936a0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a59-9500000000-9bbbd7b252fb9a0b438d | View in MoNA |
|---|
|
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|