| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:48:55 -0600 |
|---|
| Update Date | 2015-09-08 17:48:55 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
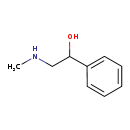
| Name: | N-Methylphenylethanolamine |
|---|
| Description | |
|---|
| Structure | |
|---|
| Synonyms: | | Value | Source |
|---|
| (+-)-alpha-((Methylamino)methyl)benzenemethanol | ChEBI | | (+-)-Halostachine | ChEBI | | alpha-[(Methylamino)methyl]-benzyl alcohol | Kegg | | (+-)-a-((Methylamino)methyl)benzenemethanol | Generator | | (+-)-Α-((methylamino)methyl)benzenemethanol | Generator | | a-[(Methylamino)methyl]-benzyl alcohol | Generator | | Α-[(methylamino)methyl]-benzyl alcohol | Generator | | MPEOA | MeSH | | N-Methylphenylethanolamine hydrochloride | MeSH | | N-Methylphenylethanolamine hydrochloride, (+-)-isomer | MeSH | | N-Methylphenylethanolamine, (+-)-isomer | MeSH | | N-Methylphenylethanolamine, (R)-isomer | MeSH | | 2-(methylamino)-1-Phenylethanol | HMDB | | 2-methylamino-1-Phenylethanol | HMDB | | alpha-((methylamino)Methyl)-DL-benzyl alcohol | HMDB | | alpha-(Methylaminomethyl)benzyl alcohol | HMDB | | DL-alpha-(Methylaminomethyl)benzyl alcohol | HMDB | | Halostachine | HMDB |
|
|---|
| Chemical Formula: | C9H13NO |
|---|
| Weight: | Average: 151.2056
Monoisotopic: 151.099714043 |
|---|
| InChI Key: | ZCTYHONEGJTYQV-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C9H13NO/c1-10-7-9(11)8-5-3-2-4-6-8/h2-6,9-11H,7H2,1H3 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 2-(methylamino)-1-phenylethan-1-ol |
|---|
| Traditional IUPAC Name: | halostachine |
|---|
| SMILES: | CNCC(O)C1=CC=CC=C1 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as aralkylamines. These are alkylamines in which the alkyl group is substituted at one carbon atom by an aromatic hydrocarbyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | Aralkylamines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Aralkylamine
- Benzenoid
- Monocyclic benzene moiety
- Secondary alcohol
- 1,2-aminoalcohol
- Secondary amine
- Secondary aliphatic amine
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Aromatic alcohol
- Organooxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0fbl-3900000000-3679c2e020c8e555350d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-014i-0900000000-26f7b8d9b9810cdaec56 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-0fbl-3900000000-3679c2e020c8e555350d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-014i-0900000000-26f7b8d9b9810cdaec56 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0006-9200000000-fa15efabc5997da6e3d2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-0006-9310000000-f429ae967f272b568609 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0f89-0900000000-b348599f4b04bdf562c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0f89-0900000000-f90dd7fab94626e11828 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0zfr-7900000000-f9602e64157769f32898 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0udi-0900000000-811f3df9a97a473ef173 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0f89-2900000000-dea7a6b68785d129c556 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-056r-9500000000-1250d1db8db14b171869 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ufr-3900000000-0f2b52640015d34b8f97 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0fb9-8900000000-e361fe87072f77e77c4d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fbc-9600000000-cedaced89cfcbb8f5289 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-3900000000-61e18a02be7d3706c2a2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0006-9100000000-89cd36302e4945d395eb | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0006-9100000000-bf697c44241fe58f470e | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|