| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:48:33 -0600 |
|---|
| Update Date | 2015-09-08 17:48:33 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Liothyronine |
|---|
| Description | |
|---|
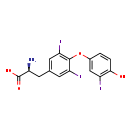
| Structure | |
|---|
| Synonyms: | | Value | Source |
|---|
| 3,5,3'-Triiodo-L-thyronine | ChEBI | | 3,5,3'-Triiodothyronine | ChEBI | | 3,5,3'TRIIODOTHYRONINE | ChEBI | | 4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodo-L-phenylalanine | ChEBI | | L-3,5,3'-Triiodothyronine | ChEBI | | L-T3 | ChEBI | | Liothyroninum | ChEBI | | Liotironina | ChEBI | | O-(4-Hydroxy-3-iodophenyl)-3,5-diiodo-L-tyrosine | ChEBI | | T3 | ChEBI | | Tertroxin | ChEBI | | Tresitope | ChEBI | | Triiodothyronine | ChEBI | | 3,3',5-Triiodo-L-thyronine | Kegg | | Thyrolar | Kegg | | 3,3',5-Triiodothyronine | HMDB | | Cytomel | HMDB | | T3 Thyroid hormone | HMDB | | Thyroid hormone, T3 | HMDB | | Liothyronine sodium | HMDB | | 3,3',5'-Triiodo-L-thyronine | HMDB | | 3,3',5'-Triiodothyronine | HMDB | | 4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenylalanine | HMDB | | Cyronine | HMDB | | L-3,3',5-Triiodo-thyronine | HMDB | | L-3,3',5-Triiodothyronine | HMDB | | L-3-[4-(4-Hydroxy-3-iodophenoxy)-3,5-diiodophenyl]-alanine | HMDB | | L-Liothyronine | HMDB | | L-Triiodothyronine | HMDB | | Liothyronin | HMDB | | Triiodo-L-thyronine | HMDB |
|
|---|
| Chemical Formula: | C15H12I3NO4 |
|---|
| Weight: | Average: 650.9735
Monoisotopic: 650.790038137 |
|---|
| InChI Key: | AUYYCJSJGJYCDS-LBPRGKRZSA-N |
|---|
| InChI: | InChI=1S/C15H12I3NO4/c16-9-6-8(1-2-13(9)20)23-14-10(17)3-7(4-11(14)18)5-12(19)15(21)22/h1-4,6,12,20H,5,19H2,(H,21,22)/t12-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2S)-2-amino-3-[4-(4-hydroxy-3-iodophenoxy)-3,5-diiodophenyl]propanoic acid |
|---|
| Traditional IUPAC Name: | liothyronine |
|---|
| SMILES: | N[C@@H](CC1=CC(I)=C(OC2=CC(I)=C(O)C=C2)C(I)=C1)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as phenylalanine and derivatives. Phenylalanine and derivatives are compounds containing phenylalanine or a derivative thereof resulting from reaction of phenylalanine at the amino group or the carboxy group, or from the replacement of any hydrogen of glycine by a heteroatom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Phenylalanine and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Phenylalanine or derivatives
- Diphenylether
- Diaryl ether
- 3-phenylpropanoic-acid
- Alpha-amino acid
- Amphetamine or derivatives
- L-alpha-amino acid
- Phenoxy compound
- 2-iodophenol
- 2-halophenol
- Phenol ether
- Iodobenzene
- 1-hydroxy-2-unsubstituted benzenoid
- Halobenzene
- Phenol
- Aralkylamine
- Aryl iodide
- Aryl halide
- Monocyclic benzene moiety
- Benzenoid
- Amino acid
- Ether
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Organic nitrogen compound
- Primary aliphatic amine
- Organohalogen compound
- Organoiodide
- Organonitrogen compound
- Organooxygen compound
- Primary amine
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Amine
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05a9-5090378000-014ef55558b58a3d38f2 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_3) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_2_4) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS ("Liothyronine,1TMS,#1" TMS) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, N/A (Annotated) | splash10-000i-0009001000-1d47041336da63b556e4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, N/A (Annotated) | splash10-000i-1009001000-15f70073b9018f013c9c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, N/A (Annotated) | splash10-000i-0009000000-71c231d7a3ab505815ff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-0udi-0000009000-f932659d1e6d8a622985 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0udi-0000009000-6506a7a71dab928abfb0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0fc0-0700119000-1df6db0e3031eb2739fa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-004i-0900000000-973fc871534b19242cc8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-004i-0900000000-ac3c4a198bb017dc7ccc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Positive | splash10-0udi-0000009000-2770ece749a9fa1042f4 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Positive | splash10-0udi-0000009000-881174c66da97022d645 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Positive | splash10-0a4i-0000019000-6f798ae76702a3053339 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Positive | splash10-0a4i-0000219000-d2ca9f0cc3c1b47176b5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Positive | splash10-056r-0021915000-6f3810619f383bce651a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0udi-0000009000-f932659d1e6d8a622985 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0udi-0000009000-6506a7a71dab928abfb0 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-0fc0-0700119000-1df6db0e3031eb2739fa | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-004i-0900000000-973fc871534b19242cc8 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , negative | splash10-004i-0900000000-ac3c4a198bb017dc7ccc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0udi-0000009000-2770ece749a9fa1042f4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0pb9-0000009000-4d60e144164273111464 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-0000149000-87babecf610c68223da5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-016r-0039010000-b57f6dd23e6456cf7c1e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0010009000-50e441d89395debe176b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-001j-0050139000-a259e39ad1204b60b373 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-00e9-9262431000-bcc1f7554c4e309cef17 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|