| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-09-08 17:48:22 -0600 |
|---|
| Update Date | 2015-09-08 17:48:22 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
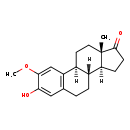
| Name: | 2-Methoxyestrone |
|---|
| Description | |
|---|
| Structure | |
|---|
| Synonyms: | | Value | Source |
|---|
| 2-(8S,9S,13S,14S)-3-Hydroxy-2-methoxy-13-methyl-7,8,9,11,12,14,15,16-octahydro-6H-cyclopenta[a]phenanthren-17-one | HMDB | | 2-Hydroxyestrone 2-methyl ether | HMDB | | 2-Methoxy-17-oxoestra-1,3,5(10)-trien-3-ol | HMDB | | 2-Methoxy-3-hydroxyestra-1,3,5(10)-trien-17-one | HMDB | | 3-Hydroxy-2-methoxy-estra-1,3,5(10)-trien-17-one | HMDB | | 3-Hydroxy-2-methoxyestra-1,3,5(10)-trien-17-one | HMDB | | Methoxy-estrone | HMDB |
|
|---|
| Chemical Formula: | C19H24O3 |
|---|
| Weight: | Average: 300.3921
Monoisotopic: 300.172544634 |
|---|
| InChI Key: | WHEUWNKSCXYKBU-QPWUGHHJSA-N |
|---|
| InChI: | InChI=1S/C19H24O3/c1-19-8-7-12-13(15(19)5-6-18(19)21)4-3-11-9-16(20)17(22-2)10-14(11)12/h9-10,12-13,15,20H,3-8H2,1-2H3/t12-,13+,15-,19-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (1S,10R,11S,15S)-5-hydroxy-4-methoxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-one |
|---|
| Traditional IUPAC Name: | (1S,10R,11S,15S)-5-hydroxy-4-methoxy-15-methyltetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadeca-2(7),3,5-trien-14-one |
|---|
| SMILES: | [H][C@@]12CCC(=O)[C@@]1(C)CC[C@]1([H])C3=C(CC[C@@]21[H])C=C(O)C(OC)=C3 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as estrogens and derivatives. These are steroids with a structure containing a 3-hydroxylated estrane. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Steroids and steroid derivatives |
|---|
| Sub Class | Estrane steroids |
|---|
| Direct Parent | Estrogens and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Estrogen-skeleton
- 3-hydroxysteroid
- Oxosteroid
- 17-oxosteroid
- Hydroxysteroid
- Phenanthrene
- Tetralin
- Anisole
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- Benzenoid
- Ketone
- Ether
- Organic oxygen compound
- Organooxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic homopolycyclic compound
|
|---|
| Molecular Framework | Aromatic homopolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-0v00-3962100000-dc012225743e8174ee72 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (1 MEOX; 1 TMS) | splash10-0ufr-4962100000-5be9e66baad9de695043 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0v00-3962100000-dc012225743e8174ee72 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-0ufr-4962100000-5be9e66baad9de695043 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0fg9-1390000000-61eb2b2fc7bb1d35c435 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-072c-1059000000-ebdce86a42908f5f5ea5 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_1) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (TBDMS_1_2) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Positive (Annotated) | splash10-0udi-0119000000-0bdc31d3bf6c50adcb9b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Positive (Annotated) | splash10-000i-0920000000-5046a6edcda6f0db50bb | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Positive (Annotated) | splash10-0fa9-1900000000-dcf45f9d507508937962 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0129000000-5f9334f16cca6272fe08 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0g4i-0793000000-494025fc807309cad403 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0fe0-5690000000-938674197a273bfc325f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-141b03af24d67c29ebc0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-5f7c58555ff69013e200 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-055f-1090000000-88737d24caa85f3a6622 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0029000000-df48a14f457e57ae7b36 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0zir-1493000000-8cc03c225697e74b97e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f97-3920000000-38e67b46e7413078ae09 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0090000000-dd82c6133109d0e6e28e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-0090000000-03c0532ee78fc6f18ec6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0159-0190000000-1cadf82cc0f992845f31 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|