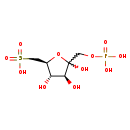| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-06-30 15:22:53 -0600 |
|---|
| Update Date | 2015-08-05 16:22:05 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | 6-Deoxy-6-sulfo-D-fructose 1-phosphate |
|---|
| Description | A carbohydrate sulfonate that is D-fructofuranose-1-phosphate in which the hydroxy group at at position 6 is replaced by a sulfo group |
|---|
| Structure | |
|---|
| Synonyms: | - 6-Deoxy-6-sulfO-D-fructofuranose 1-phosphate
- 6-Deoxy-6-sulfO-D-fructofuranose 1-phosphoric acid
- 6-Deoxy-6-sulfO-D-fructose 1-phosphate
- 6-Deoxy-6-sulfO-D-fructose 1-phosphoric acid
- 6-Deoxy-6-sulfofructose 1-phosphate
- 6-Deoxy-6-sulfofructose 1-phosphoric acid
- 6-Deoxy-6-sulphO-D-fructofuranose 1-phosphate
- 6-Deoxy-6-sulphO-D-fructofuranose 1-phosphoric acid
- 6-Deoxy-6-sulphO-D-fructose 1-phosphate
- 6-Deoxy-6-sulphO-D-fructose 1-phosphoric acid
- 6-Deoxy-6-sulphofructose 1-phosphate
- 6-Deoxy-6-sulphofructose 1-phosphoric acid
|
|---|
| Chemical Formula: | C6H13O11PS |
|---|
| Weight: | Average: 324.19
Monoisotopic: 323.991619411 |
|---|
| InChI Key: | IZVMCURFIBVEOJ-VRPWFDPXSA-N |
|---|
| InChI: | InChI=1S/C6H13O11PS/c7-4-3(1-19(13,14)15)17-6(9,5(4)8)2-16-18(10,11)12/h3-5,7-9H,1-2H2,(H2,10,11,12)(H,13,14,15)/t3-,4-,5+,6?/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | [(2S,3S,4S)-3,4,5-trihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]methanesulfonic acid |
|---|
| Traditional IUPAC Name: | [(2S,3S,4S)-3,4,5-trihydroxy-5-[(phosphonooxy)methyl]oxolan-2-yl]methanesulfonic acid |
|---|
| SMILES: | O[C@H]1[C@H](O)C(O)(COP(O)(O)=O)O[C@@H]1CS(O)(=O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Pentose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentose phosphate
- Pentose-5-phosphate
- C-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Monoalkyl phosphate
- Sugar acid
- Organic phosphoric acid derivative
- Alkyl phosphate
- Phosphoric acid ester
- Alkanesulfonic acid
- Tetrahydrofuran
- Sulfonyl
- Organosulfonic acid
- Organic sulfonic acid or derivatives
- Organosulfonic acid or derivatives
- 1,2-diol
- Secondary alcohol
- Hemiacetal
- Organoheterocyclic compound
- Oxacycle
- Polyol
- Alcohol
- Organosulfur compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -3 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 77263 | | HMDB ID | Not Available | | Pubchem Compound ID | 86289122 | | Kegg ID | C20831 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|