| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-06-08 15:27:29 -0600 |
|---|
| Update Date | 2015-09-14 16:46:24 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | alpha-D-Aldose 1-phosphate |
|---|
| Description | In E. coli, alpha-D-Aldose 1-phosphate is involved in several reactions. ADP-sugar pyrophosphatase catalyzes the reaction an ADP-sugar + H2O → AMP + an alpha-D-aldose 1-phosphate; UDP-sugar pyrophosphorylase catalyzes the reaction an alpha-D-aldose 1-phosphate + a nucleoside diphosphate + H+ = a nucleotide diphosphate-aldose + phosphate; The reaction a UDP-sugar[periplasmic space] + H2O[periplasmic space] → UMP[periplasmic space] + an alpha-D-aldose 1-phosphate[periplasmic space] + 2 H+[periplasmic space] is catalyzed by UDP-sugar hydrolase (BioCyc compound class: Alpha-D-aldose-1-phosphates). |
|---|
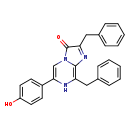
| Structure | |
|---|
| Synonyms: | - a-D-Aldose 1-phosphate
- a-D-Aldose 1-phosphoric acid
- alpha-D-Aldose 1-phosphoric acid
- Renillluciferin
- α-D-Aldose 1-phosphate
- α-D-Aldose 1-phosphoric acid
|
|---|
| Chemical Formula: | C26H21N3O2 |
|---|
| Weight: | Average: 407.473
Monoisotopic: 407.163376928 |
|---|
| InChI Key: | KAEGGIFPLJZUOZ-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C26H21N3O2/c30-21-13-11-20(12-14-21)24-17-29-25(22(27-24)15-18-7-3-1-4-8-18)28-23(26(29)31)16-19-9-5-2-6-10-19/h1-14,17,27,30H,15-16H2 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 2,8-dibenzyl-6-(4-hydroxyphenyl)-3H,7H-imidazo[1,2-a]pyrazin-3-one |
|---|
| Traditional IUPAC Name: | renilla luciferin |
|---|
| SMILES: | OC1=CC=C(C=C1)C1=CN2C(=O)C(CC3=CC=CC=C3)=NC2=C(CC2=CC=CC=C2)N1 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as imidazopyrazines. These are organic heteropolycyclic compounds containing a pyrazine ring fused to an imidazole ring. These also include hydrogenated derivatives of the imidazopyrazine moiety. Imidazole is 5-membered ring consisting of three carbon atoms, and two nitrogen centers at the 1- and 3-positions. Pyrazine is a 6-membered ring consisting of six carbon atoms and two nitrogen centers at ring positions 1 and 4. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Imidazopyrazines |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Imidazopyrazines |
|---|
| Alternative Parents | |
|---|
| Substituents | - Imidazopyrazine
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Monocyclic benzene moiety
- N-substituted imidazole
- Pyrazine
- Benzenoid
- Imidazole
- Azole
- Heteroaromatic compound
- Lactam
- Azacycle
- Organic oxygen compound
- Organic nitrogen compound
- Organooxygen compound
- Organonitrogen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 16531 | | HMDB ID | Not Available | | Pubchem Compound ID | 2762722 | | Kegg ID | C00991 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|