| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-06-04 16:16:58 -0600 |
|---|
| Update Date | 2015-08-05 16:22:04 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
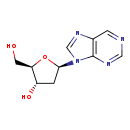
| Name: | Purine deoxyribonucleoside |
|---|
| Description | A deoxyribonucleoside containing a purine base |
|---|
| Structure | |
|---|
| Synonyms: | - 2'-Deoxy-N-b-D-ribosylpurine
- 2'-Deoxy-N-beta-D-ribosylpurine
- 2'-Deoxy-N-β-D-ribosylpurine
- 2'-Deoxynebularine
- 2-Deoxy-N-D-ribosylpurine
- 6-deamino-2'-Deoxyadenosine
- 9-(2-Deoxy-b-D-ribofuranosyl)-9H-purine
- 9-(2-Deoxy-beta-D-ribofuranosyl)-9H-purine
- 9-(2-Deoxy-β-D-ribofuranosyl)-9H-purine
- 9-(b-D-2'-Deoxyribofuranosyl)purine
- 9-(beta-D-2'-Deoxyribofuranosyl)purine
- 9-(β-D-2'-deoxyribofuranosyl)purine
- N-(b-D-2'-Deoxyribosyl)purine
- N-(beta-D-2'-Deoxyribosyl)purine
- N-(β-D-2'-deoxyribosyl)purine
|
|---|
| Chemical Formula: | C10H12N4O3 |
|---|
| Weight: | Average: 236.231
Monoisotopic: 236.090940262 |
|---|
| InChI Key: | WJBNIBFTNGZFBW-DJLDLDEBSA-N |
|---|
| InChI: | InChI=1S/C10H12N4O3/c15-3-8-7(16)1-9(17-8)14-5-13-6-2-11-4-12-10(6)14/h2,4-5,7-9,15-16H,1,3H2/t7-,8+,9+/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2R,3S,5R)-2-(hydroxymethyl)-5-(9H-purin-9-yl)oxolan-3-ol |
|---|
| Traditional IUPAC Name: | (2R,3S,5R)-2-(hydroxymethyl)-5-(purin-9-yl)oxolan-3-ol |
|---|
| SMILES: | OC[C@H]1O[C@H](C[C@@H]1O)N1C=NC2=C1N=CN=C2 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as purine 2'-deoxyribonucleosides. Purine 2'-deoxyribonucleosides are compounds consisting of a purine linked to a ribose which lacks a hydroxyl group at position 2. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Purine nucleosides |
|---|
| Sub Class | Purine 2'-deoxyribonucleosides |
|---|
| Direct Parent | Purine 2'-deoxyribonucleosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Purine 2'-deoxyribonucleoside
- Imidazopyrimidine
- Purine
- N-substituted imidazole
- Pyrimidine
- Tetrahydrofuran
- Heteroaromatic compound
- Azole
- Imidazole
- Secondary alcohol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Alcohol
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 72810 | | HMDB ID | Not Available | | Pubchem Compound ID | 65148 | | Kegg ID | C20463 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|