| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-06-04 15:17:29 -0600 |
|---|
| Update Date | 2015-09-14 16:46:21 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | L-Alanyl-tRNA |
|---|
| Description | NULL |
|---|
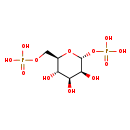
| Structure | |
|---|
| Synonyms: | - Man1,6BP
- Mannose-1,6-bisphosphate
- Mannose-1,6-bisphosphate, (a-D)-isomer
- Mannose-1,6-bisphosphate, (alpha-D)-isomer
- Mannose-1,6-bisphosphate, (L)-isomer
- Mannose-1,6-bisphosphate, (α-D)-isomer
- Mannose-1,6-bisphosphoric acid
- Mannose-1,6-bisphosphoric acid, (a-D)-isomer
- Mannose-1,6-bisphosphoric acid, (alpha-D)-isomer
- Mannose-1,6-bisphosphoric acid, (L)-isomer
- Mannose-1,6-bisphosphoric acid, (α-D)-isomer
|
|---|
| Chemical Formula: | C6H14O12P2 |
|---|
| Weight: | Average: 340.114
Monoisotopic: 339.996049887 |
|---|
| InChI Key: | RWHOZGRAXYWRNX-RWOPYEJCSA-N |
|---|
| InChI: | InChI=1S/C6H14O12P2/c7-3-2(1-16-19(10,11)12)17-6(5(9)4(3)8)18-20(13,14)15/h2-9H,1H2,(H2,10,11,12)(H2,13,14,15)/t2-,3-,4+,5+,6-/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | {[(2R,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(phosphonooxy)oxan-2-yl]methoxy}phosphonic acid |
|---|
| Traditional IUPAC Name: | [(2R,3S,4S,5S,6R)-3,4,5-trihydroxy-6-(phosphonooxy)oxan-2-yl]methoxyphosphonic acid |
|---|
| SMILES: | O[C@H]1[C@H](O)[C@@H](COP(O)(O)=O)O[C@H](OP(O)(O)=O)[C@H]1O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as hexose phosphates. These are carbohydrate derivatives containing a hexose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Hexose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Hexose phosphate
- Monosaccharide phosphate
- Monoalkyl phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Organic oxide
- Hydrocarbon derivative
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -4 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | - Aminoacyl-tRNA biosynthesis ec00970
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | C00886 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|