| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2015-06-04 15:14:56 -0600 |
|---|
| Update Date | 2015-09-17 16:25:05 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | L-Lysyl-tRNA |
|---|
| Description | L-Lysyl-tRNA is an intermediate in tRNA charging pathway in E.coli. It is a product for the enzyme lysyl-tRNA synthetase which catalyzes the reaction ATP + L-lysine + tRNALys -> AMP + diphosphate + L-lysyl-tRNALys (BioCyc class: Charged-LYS-tRNAs). |
|---|
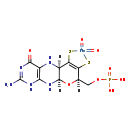
| Structure | |
|---|
| Synonyms: | - moco (dioxyo)
- Molybdenum cofactor
- Molybdenum cofactor (dioxyo)
- Molybdopterin
- Molybdopterin cofactor
- MoO2(OH)DTPP-MP
- Pterin molybdenum cofactor
|
|---|
| Chemical Formula: | C10H16MoN5O8PS2 |
|---|
| Weight: | Average: 525.31
Monoisotopic: 526.923197 |
|---|
| InChI Key: | VUKICSJFFDCESC-UHFFFAOYSA-L |
|---|
| InChI: | InChI=1S/C10H14N5O6PS2.Mo.2H2O/c11-10-14-7-4(8(16)15-10)12-3-6(24)5(23)2(21-9(3)13-7)1-20-22(17,18)19;;;/h2-3,9,12,23-24H,1H2,(H2,17,18,19)(H4,11,13,14,15,16);;2*1H2/q;+2;;/p-2 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | {[(1R,10R,16R)-5-amino-7,13,13-trioxo-17-oxa-12,14-dithia-2,4,6,9-tetraaza-13-molybdatetracyclo[8.7.0.0³,⁸.0¹¹,¹⁵]heptadeca-3(8),5,11(15)-trien-16-yl]methoxy}phosphonic acid |
|---|
| Traditional IUPAC Name: | [(1R,10R,16R)-5-amino-7,13,13-trioxo-17-oxa-12,14-dithia-2,4,6,9-tetraaza-13-molybdatetracyclo[8.7.0.0³,⁸.0¹¹,¹⁵]heptadeca-3(8),5,11(15)-trien-16-yl]methoxyphosphonic acid |
|---|
| SMILES: | O.O.[Mo++].[H]C1(COP(O)([O-])=O)OC2([H])NC3=C(NC2([H])C(S)=C1S)C([O-])=NC(=N)N3 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as molybdopterins. These are cofactors or analogs thereof, with a structure based on a furan ring fused to a pterin. In addition, the pyran ring features two thiolates, which serve as ligands in molybdo- and tungstoenzymes. In some cases, the alkyl phosphate group is replaced by an alkyl diphosphate nucleotide. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Molybdopterins |
|---|
| Alternative Parents | |
|---|
| Substituents | - Molybdopterin
- Pyranopterin
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Pyran
- Pyrimidine
- Alkyl phosphate
- Heteroaromatic compound
- Vinylogous amide
- Oxacycle
- Azacycle
- Organic transition metal salt
- Secondary amine
- Organic nitrogen compound
- Amine
- Primary amine
- Hydrocarbon derivative
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxide
- Organic salt
- Organic zwitterion
- Organic oxygen compound
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | - Aminoacyl-tRNA biosynthesis ec00970
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 71306 | | HMDB ID | Not Available | | Pubchem Compound ID | 56928099 | | Kegg ID | C01931 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|