| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 13:28:12 -0600 |
|---|
| Update Date | 2015-12-09 14:08:04 -0700 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
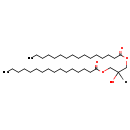
| Name: | DG(16:0/16:0/0:0) |
|---|
| Description | DG(16:0/16:0/0:0) belongs to the family of Diacylglycerols. These are glycerolipids lipids containing a common glycerol backbone to which at least one fatty acyl group is esterified. DG(16:0/16:0/0:0) is also a substrate of diacylglycerol kinase. It is involved in the phospholipid metabolic pathway. |
|---|
| Structure | |
|---|
| Synonyms: | - 1,2-dihexadecanoyl-rac-glycerol
- 1,2-dipalmitoyl-rac-glycerol
- DAG(16:0/16:0)
- DAG(32:0)
- DG(16:0/16:0)
- DG(32:0)
- Diacylglycerol
- Diacylglycerol(16:0/16:0)
- Diacylglycerol(32:0)
- Diglyceride
|
|---|
| Chemical Formula: | C35H68O5 |
|---|
| Weight: | Average: 568.9114
Monoisotopic: 568.506675286 |
|---|
| InChI Key: | GFAZGHREJPXDMH-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C35H68O5/c1-3-5-7-9-11-13-15-17-19-21-23-25-27-29-34(37)39-31-33(36)32-40-35(38)30-28-26-24-22-20-18-16-14-12-10-8-6-4-2/h33,36H,3-32H2,1-2H3 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 3-(hexadecanoyloxy)-2-hydroxypropyl hexadecanoate |
|---|
| Traditional IUPAC Name: | 3-(hexadecanoyloxy)-2-hydroxypropyl hexadecanoate |
|---|
| SMILES: | CCCCCCCCCCCCCCCC(=O)OCC(O)COC(=O)CCCCCCCCCCCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,3-diacylglycerols. These are diacylglycerols containing a glycerol acylated at positions 1 and 3. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Glycerolipids |
|---|
| Sub Class | Diradylglycerols |
|---|
| Direct Parent | 1,3-diacylglycerols |
|---|
| Alternative Parents | |
|---|
| Substituents | - 1,3-acyl-sn-glycerol
- Fatty acid ester
- Fatty acyl
- Dicarboxylic acid or derivatives
- Secondary alcohol
- Carboxylic acid ester
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (1 TMS) - 70eV, Positive | splash10-009i-9442002000-1c70e6cdb9d0059c9f78 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-014i-1164090000-f61c5a8687070d32034f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03ds-3593120000-1d4e9a2895d78194c6ea | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000b-3960300000-18ebc7c42009a935f42c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0ap0-0091040000-f87df3a9edff9da54159 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-1091000000-ae622c34c161eca19027 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4r-3090000000-140c0a7e836fa18c5835 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-02t9-2145390000-29d316fbbfc29fddb910 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0hti-9253430000-f85adc23f2f603cc2303 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-06rg-9431000000-da30c3887845539a207c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-014i-0054090000-657b54d3b8edcbfabb3e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0a4r-2093020000-fc77918b8536842e1159 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a5i-0090000000-b8e34fb91883d8a80048 | View in MoNA |
|---|
|
|---|
| References |
|---|
| References: | - Yurtsever D. (2007). Fatty acid methyl ester profiling of Enterococcus and Esherichia coli for microbial source tracking. M.sc. Thesis. Villanova University: U.S.A
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 77619 | | HMDB ID | HMDB07098 | | Pubchem Compound ID | 68149 | | Kegg ID | Not Available | | ChemSpider ID | 61457 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|