Structural search and advanced query search is temporarily unavailable. We are working to fix this issue. Thank you for your support and patience.
pantotheine 4'-phosphate (M2MDB003606)
| Record Information | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Version | 2.0 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Creation Date | 2012-10-10 12:22:28 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Update Date | 2015-09-13 15:15:34 -0600 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Secondary Accession Numbers |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Identification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
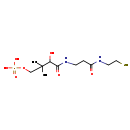
| Name: | pantotheine 4'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | A phosphopantetheine that has formula C11H23N2O7PS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Structure | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synonyms: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Formula: | C11H23N2O7PS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Weight: | Average: 358.348 Monoisotopic: 358.096358302 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI Key: | JDMUPRLRUUMCTL-UHFFFAOYSA-N | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| InChI: | InChI=1S/C11H23N2O7PS/c1-11(2,7-20-21(17,18)19)9(15)10(16)13-4-3-8(14)12-5-6-22/h9,15,22H,3-7H2,1-2H3,(H,12,14)(H,13,16)(H2,17,18,19) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number: | 2226-71-3 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| IUPAC Name: | [3-hydroxy-2,2-dimethyl-3-({2-[(2-sulfanylethyl)carbamoyl]ethyl}carbamoyl)propoxy]phosphonic acid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Traditional IUPAC Name: | pantetheine 4'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES: | CC(C)(COP(O)(O)=O)C(O)C(=O)NCCC(=O)NCCS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemical Taxonomy | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Description | belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Kingdom | Organic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Super Class | Organic acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Class | Organic phosphoric acids and derivatives | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sub Class | Phosphate esters | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Direct Parent | Monoalkyl phosphates | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Alternative Parents | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Substituents |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molecular Framework | Aliphatic acyclic compounds | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Descriptors |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| State: | Solid | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Charge: | -2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Experimental Properties: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Predicted Properties |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Biological Properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Cellular Locations: | Cytoplasm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reactions: | 4-Phosphopantothenoylcysteine + Hydrogen ion <> Carbon dioxide + Pantetheine 4'-phosphate + pantotheine 4'-phosphate 4-Phosphopantothenoylcysteine > pantotheine 4'-phosphate + Carbon dioxide Adenosine triphosphate + pantotheine 4'-phosphate > Pyrophosphate + 3'-dephospho-CoA Pantetheine + Adenosine triphosphate + Pantetheine > Pantetheine 4'-phosphate + Adenosine diphosphate + pantotheine 4'-phosphate + ADP 4-Phosphopantothenoylcysteine > Pantetheine 4'-phosphate + Carbon dioxide + pantotheine 4'-phosphate Pantetheine 4'-phosphate + Adenosine triphosphate + pantotheine 4'-phosphate > Dephospho-CoA + Pyrophosphate Dephospho-CoA + Water <> Pantetheine 4'-phosphate + Adenosine monophosphate + pantotheine 4'-phosphate | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMPDB Pathways: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| KEGG Pathways: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EcoCyc Pathways: | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Concentrations | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Not Available | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Spectra: | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| References: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Synthesis Reference: | Masuda, Toru; Fujii, Shoichiro; Takanohashi, Kunio. Pantetheine 4'-phosphate. Jpn. Tokkyo Koho (1971), 3 pp. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Material Safety Data Sheet (MSDS) | Not Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Links | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| External Links: |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Enzymes
- General function:
- Involved in catalytic activity
- Specific function:
- Reversibly transfers an adenylyl group from ATP to 4'- phosphopantetheine, yielding dephospho-CoA (dPCoA) and pyrophosphate
- Gene Name:
- coaD
- Uniprot ID:
- P0A6I6
- Molecular weight:
- 17837
Reactions
| ATP + pantetheine 4'-phosphate = diphosphate + 3'-dephospho-CoA. |
- General function:
- Involved in dephospho-CoA kinase activity
- Specific function:
- Catalyzes the phosphorylation of the 3'-hydroxyl group of dephosphocoenzyme A to form coenzyme A
- Gene Name:
- coaE
- Uniprot ID:
- P0A6I9
- Molecular weight:
- 22622
Reactions
| ATP + 3'-dephospho-CoA = ADP + CoA. |
- General function:
- Involved in phosphopantothenate--cysteine ligase activity
- Specific function:
- Catalyzes two steps in the biosynthesis of coenzyme A. In the first step cysteine is conjugated to 4'-phosphopantothenate to form 4-phosphopantothenoylcysteine, in the latter compound is decarboxylated to form 4'-phosphopantotheine
- Gene Name:
- coaBC
- Uniprot ID:
- P0ABQ0
- Molecular weight:
- 43438
Reactions
| N-((R)-4'-phosphopantothenoyl)-L-cysteine = pantotheine 4'-phosphate + CO(2). |
| CTP + (R)-4'-phosphopantothenate + L-cysteine = CMP + diphosphate + N-((R)-4'-phosphopantothenoyl)-L-cysteine. |