| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:22:11 -0600 |
|---|
| Update Date | 2015-06-03 17:26:10 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | TDP |
|---|
| Description | Thymidine diphosphate is a nucleoside diphosphate. It is an ester of pyrophosphoric acid with the nucleoside thymidine. TDP consists of the pyrophosphate group, the pentose sugar ribose, and the nucleobase thymine. TDP is produced during the synthesis of LPS via the enzyme 4-alpha-L-fucosyltransferase which catalyzes the reaction TDP-Fuc4NAc + Und-PP-GlcNAc-ManNAcA = TDP + Und-PP-GlcNAc-ManNAcA-Fuc4NAc. This reaction leads to the Lipid III product, which is third lipid-linked intermediate involved in LPS synthesis. |
|---|
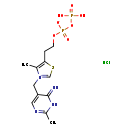
| Structure | |
|---|
| Synonyms: | - 2-3-(4-amino-2-methylpyrimidin-5-yl)methyl-4-methyl-1,3-thiazol-3-ium-5-ylethyl phosphono hydrogen phosphate chloride
- 2-3-(4-amino-2-Methylpyrimidin-5-yl)methyl-4-methyl-1,3-thiazol-3-ium-5-ylethyl phosphono hydrogen phosphoric acid chloride
- 3-(4-amino-2-methylpyrimidin-5-yl)methyl-5-(2-diphosphoethyl)-4-methyl-1,3-thiazolium chloride
- Cocarboxilasa
- Cocarboxylase
- Cocarboxylasum
- Coenzymate
- Coenzymic acid
- Thiamine diphosphate chloride
- Thiamine diphosphoric acid chloride
- Thiamine pyrophosphate
- Thiamine pyrophosphoric acid
- Thiazolium, 3-((4-amino-2-methyl-5-pyrimidinyl)methyl)-4-methyl-5-(4,6,6-trihydroxy-3,5-dioxa-4,6-diphosphahex-1-yl)-, chloride, P,P'-dioxide
|
|---|
| Chemical Formula: | C12H19ClN4O7P2S |
|---|
| Weight: | Average: 460.767
Monoisotopic: 460.013820403 |
|---|
| InChI Key: | YXVCLPJQTZXJLH-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C12H18N4O7P2S.ClH/c1-8-11(3-4-22-25(20,21)23-24(17,18)19)26-7-16(8)6-10-5-14-9(2)15-12(10)13;/h5,7H,3-4,6H2,1-2H3,(H4-,13,14,15,17,18,19,20,21);1H |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 3-[(6-imino-2-methyl-1,6-dihydropyrimidin-5-yl)methyl]-4-methyl-5-{2-[(phosphono phosphonato)oxy]ethyl}-1,3-thiazol-3-ium hydrochloride |
|---|
| Traditional IUPAC Name: | 3-[(4-imino-2-methyl-3H-pyrimidin-5-yl)methyl]-4-methyl-5-{2-[(phosphono phosphonato)oxy]ethyl}-1,3-thiazol-3-ium hydrochloride |
|---|
| SMILES: | Cl.CC1=C(CCOP([O-])(=O)OP(O)(O)=O)SC=[N+]1CC1=CN=C(C)NC1=N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Thiamine phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiamine-phosphate
- Organic pyrophosphate
- 4,5-disubstituted 1,3-thiazole
- Aminopyrimidine
- Monoalkyl phosphate
- Organic phosphoric acid derivative
- Phosphoric acid ester
- Alkyl phosphate
- Imidolactam
- Heteroaromatic compound
- Azole
- Thiazole
- Azacycle
- Organic oxygen compound
- Organic chloride salt
- Organic oxide
- Primary amine
- Amine
- Organooxygen compound
- Organonitrogen compound
- Organopnictogen compound
- Organic nitrogen compound
- Hydrocarbon derivative
- Organic zwitterion
- Organic salt
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 18290 | | HMDB ID | Not Available | | Pubchem Compound ID | 9068 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | TDP | | BioCyc ID | Not Available |
|
|---|