| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:22:01 -0600 |
|---|
| Update Date | 2015-06-03 17:26:09 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | 2-(3,4-dihydroxybenzoyloxy)-4,6-dihydroxybenzoate |
|---|
| Description | 2-(3,4-dihydroxybenzoyloxy)-4,6-dihydroxybenzoate is an intermediate in the degradation of quercetin by E. coli. Quercetin is a flavonoid widely distributed in many plants and fruits including red grapes, citrus fruit, tomato, broccoli and other leafy green vegetables, and a number of berries, including raspberries and cranberries. In E. coli it is metabolized by Quercetin 2,3-dioxygenase. This enzyme catalyzes the reaction Quercetin + O2 = 2-(3,4-dihydroxybenzoyloxy)-4,6-dihydroxybenzoate + CO + H+. Quercetin is not a normal growth substrate for E. coli but it is found in high levels in the human gut. This enzyme may have evolved to break down quercitin to prevent its inhibition of key E. coli proteins, such as DNA gyrase. |
|---|
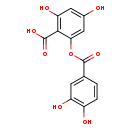
| Structure | |
|---|
| Synonyms: | - 2,4DiOH-6(2,4DiOHBenAcid)BenzAcid
- 2-(3,4-Dihydroxybenzoyl)oxy-4,6-dihydroxybenzoate
- 2-(3,4-Dihydroxybenzoyl)oxy-4,6-dihydroxybenzoic acid
- 2-(3,4-Dihydroxybenzoyloxy)-4,6-dihydroxybenzoate
- 2-(3,4-Dihydroxybenzoyloxy)-4,6-dihydroxybenzoic acid
- 2-Protocatechoylphloroglucinolcarboxylate
- 2-Protocatechoylphloroglucinolcarboxylic acid
- 2-Protocatechuoyl phloroglucinolcarboxylate
- 2-Protocatechuoyl phloroglucinolcarboxylic acid
|
|---|
| Chemical Formula: | C14H10O8 |
|---|
| Weight: | Average: 306.2244
Monoisotopic: 306.037567296 |
|---|
| InChI Key: | GRXIELRCPYIEQI-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C14H10O8/c15-7-4-10(18)12(13(19)20)11(5-7)22-14(21)6-1-2-8(16)9(17)3-6/h1-5,15-18H,(H,19,20) |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 2-(3,4-dihydroxybenzoyloxy)-4,6-dihydroxybenzoic acid |
|---|
| Traditional IUPAC Name: | 2-(3,4-dihydroxybenzoyloxy)-4,6-dihydroxybenzoic acid |
|---|
| SMILES: | OC(=O)C1=C(O)C=C(O)C=C1OC(=O)C1=CC(O)=C(O)C=C1 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as depsides and depsidones. These are polycyclic compounds that is either a polyphenolic compound composed of two or more monocyclic aromatic units linked by an ester bond (depside), or a compound containing the depsidone structure (depsidone). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Depsides and depsidones |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Depsides and depsidones |
|---|
| Alternative Parents | |
|---|
| Substituents | - Depside backbone
- M-hydroxybenzoic acid ester
- P-hydroxybenzoic acid ester
- Dihydroxybenzoic acid
- Hydroxybenzoic acid
- Phenol ester
- Salicylic acid or derivatives
- Salicylic acid
- Benzoate ester
- Benzoic acid
- Benzoic acid or derivatives
- Phenoxy compound
- Resorcinol
- Catechol
- Benzoyl
- Phenol
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Benzenoid
- Dicarboxylic acid or derivatives
- Monocyclic benzene moiety
- Vinylogous acid
- Carboxylic acid ester
- Carboxylic acid derivative
- Carboxylic acid
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organic oxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-000i-0900000000-f063a50e2100260c680c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (5 TMS) - 70eV, Positive | splash10-001i-0090011000-57d471b57e310ee50676 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0596000000-47902ef3296c1477004b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0961000000-40a68d3f3894906dba7a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0019-5910000000-8ede257c8c7d14f1a891 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-08fr-0194000000-f4af9d9f87266faff042 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-03di-0290000000-38a97201178b58d10cd5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-06a0-3920000000-6ab6758b580e79a6a45d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0692000000-5556817bba47ece0212f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0950000000-c9c6bed2b1d180ffeb22 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0f79-3930000000-ff1140404484c2ffa85f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0fb9-0900000000-95ab0e687e19e8f7f0c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-07gi-0970000000-2eaec82c823970bda725 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fbc-3930000000-658b94bc5d3ab0cd21a8 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 16068 | | HMDB ID | HMDB0059651 | | Pubchem Compound ID | 440370 | | Kegg ID | C04524 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|