| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:21:46 -0600 |
|---|
| Update Date | 2015-06-03 17:26:08 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
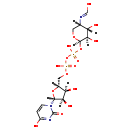
| Name: | UDP-4-Deoxy-4-formamido-beta-L-arabinose |
|---|
| Description | UDP-4-Deoxy-4-formamido-beta-L-arabinose is an intermediate in the polymixin resistance pathway. It is a substrate for the enzyme Bifunctional polymyxin resistance protein ArnA that catalyzes the oxidative decarboxylation of UDP-glucuronic acid (UDP-GlcUA) to UDP-4-keto-arabinose (UDP-Ara4O) and the addition of a formyl group to UDP-4-amino-4-deoxy-L-arabinose (UDP-L-Ara4N) to form UDP-L-4-formamido-arabinose (UDP-L-Ara4FN). The modified arabinose is attached to lipid A and is required for resistance to polymyxin and cationic antimicrobial peptides. Some Gram-negative bacteria, specifically Salmonella typhimurium and Escherichia coli, can become resistant to polymyxin by the modification of their lipid A structure via the attachment of 4-amino-4-deoxy-L-arabinopyranose (L-Ara4N) groups to one or more phosphate groups. This addition causes an absolute increase in lipid A charge, thus lowering the affinity of positively charged polymyxins. |
|---|
| Structure | |
|---|
| Synonyms: | - (2R,3S,4R,5R)-5-(2,4-Dioxo-3,4-dihydro-1(2H)-pyrimidinyl)-3,4-dihydroxytetrahydro-2-furanylmethyl (2R,3R,4S,5S)-5-formamido-3,4-dihydroxytetrahydro-2H-pyran-2-yl dihydrogen diphosphate (non-preferre
d name)
- (2R,3S,4R,5R)-5-(2,4-dioxo-3,4-dihydro-1(2H)-Pyrimidinyl)-3,4-dihydroxytetrahydro-2-furanylmethyl (2R,3R,4S,5S)-5-formamido-3,4-dihydroxytetrahydro-2H-pyran-2-yl dihydrogen diphosphoric acid (non-preferre
D name)
- (2R,3S,4R,5R)-5-(2,4-dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-ylmethoxy-hydroxyphosphoryl (2R,3R,4S,5S)-5-formamido-3,4-dihydroxyoxan-2-yl hydrogen phosphate
- (2R,3S,4R,5R)-5-(2,4-Dioxopyrimidin-1-yl)-3,4-dihydroxyoxolan-2-ylmethoxy-hydroxyphosphoryl (2R,3R,4S,5S)-5-formamido-3,4-dihydroxyoxan-2-yl hydrogen phosphoric acid
- UDP-4-Deoxy-4-formamido-b-L-arabinose
- UDP-4-Deoxy-4-formamido-β-L-arabinose
- UDP-b-L-Ara4fn
- UDP-beta-L-Ara4FN
- UDP-L-Ara4FN
- UDP-β-L-Ara4fn
- Uridine 5'-diphospho-b-(4-deoxy-4-formamido-L-arabinose)
- Uridine 5'-diphospho-beta-(4-deoxy-4-formamido-L-arabinose)
- Uridine 5'-diphospho-β-(4-deoxy-4-formamido-L-arabinose)
|
|---|
| Chemical Formula: | C15H23N3O16P2 |
|---|
| Weight: | Average: 563.3011
Monoisotopic: 563.055354727 |
|---|
| InChI Key: | QGYFHZBDXXNYAX-RTXATJJPSA-N |
|---|
| InChI: | InChI=1S/C15H23N3O16P2/c19-5-16-6-3-30-14(12(24)9(6)21)33-36(28,29)34-35(26,27)31-4-7-10(22)11(23)13(32-7)18-2-1-8(20)17-15(18)25/h1-2,5-7,9-14,21-24H,3-4H2,(H,16,19)(H,26,27)(H,28,29)(H,17,20,25)/t6-,7+,9-,10+,11+,12+,13+,14+/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | N-[(3S,4S,5R,6R)-6-({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-hydroxy-2-oxo-1,2-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-4,5-dihydroxyoxan-3-yl]carboximidic acid |
|---|
| Traditional IUPAC Name: | udp-L-ara4FN |
|---|
| SMILES: | [H][C@]1(COP(O)(=O)OP(O)(=O)O[C@@]2([H])OC[C@]([H])(N=CO)[C@]([H])(O)[C@@]2([H])O)O[C@@]([H])(N2C=CC(O)=NC2=O)[C@]([H])(O)[C@]1([H])O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrimidine ribonucleoside diphosphates. These are pyrimidine ribonucleotides with diphosphate group linked to the ribose moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine ribonucleotides |
|---|
| Direct Parent | Pyrimidine ribonucleoside diphosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidine ribonucleoside diphosphate
- Pentose phosphate
- Pentose-5-phosphate
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Organic pyrophosphate
- Pyrimidone
- Monoalkyl phosphate
- Hydropyrimidine
- Monosaccharide
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Vinylogous amide
- Tetrahydrofuran
- Heteroaromatic compound
- Urea
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- Lactam
- Azacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Oxacycle
- Organic nitrogen compound
- Alcohol
- Carbonyl group
- Organonitrogen compound
- Organooxygen compound
- Organopnictogen compound
- Organic oxide
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | Amino sugar and nucleotide sugar metabolism III | PW000895 |    | | One Carbon Pool by Folate I | PW001735 |    | | polymyxin resistance | PW002052 |    |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 47027 | | HMDB ID | Not Available | | Pubchem Compound ID | 17756768 | | Kegg ID | C16154 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|