| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:20:21 -0600 |
|---|
| Update Date | 2015-09-13 15:15:34 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Tetrahydrocurcumin |
|---|
| Description | Tetrahydrocurcumin (THC), is a product of the metabolism of curcumin by the enzyme NADPH-dependent curcumin reductase. Curcumin is a yellow, polyphenolic pigment, derived from the rhizomes of a plant (Curcuma longa Linn). It is the principal curcuminoid of the popular Indian spice turmeric, which is a member of the ginger family and is a natural antioxidant exhibiting a variety of pharmacological activities and therapeutic properties. It has long been used as a traditional medicine and as a preservative and coloring agent in foods. In E. coli curcumin is a substrate for the enzyme NADPH-dependent curcumin reductase which catalyzes the metal-independent reduction of curcumin to dihydrocurcumin (DHC) as an intermediate product, followed by further reduction to tetrahydrocurcumin (THC) as an end product. Tetrahydrocurcumin (THC) exhibits many of the same physiologic and pharmacological activities as curcumin and in some systems may exert greater antioxidant activity than curcumin [PMID:21467222] |
|---|
| Structure | |
|---|
| Synonyms: | - 1,7-bis(4-hydroxy-3-methoxyphenyl)-3,5-heptanedione
|
|---|
| Chemical Formula: | C21H24O6 |
|---|
| Weight: | Average: 372.4117
Monoisotopic: 372.1572885 |
|---|
| InChI Key: | LBTVHXHERHESKG-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C21H24O6/c1-26-20-11-14(5-9-18(20)24)3-7-16(22)13-17(23)8-4-15-6-10-19(25)21(12-15)27-2/h5-6,9-12,24-25H,3-4,7-8,13H2,1-2H3 |
|---|
| CAS number: | 36062-04-1 |
|---|
| IUPAC Name: | 1,7-bis(4-hydroxy-3-methoxyphenyl)heptane-3,5-dione |
|---|
| Traditional IUPAC Name: | tetrahydrocurcumin |
|---|
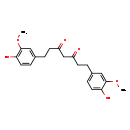
| SMILES: | COC1=CC(CCC(=O)CC(=O)CCC2=CC(OC)=C(O)C=C2)=CC=C1O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as curcuminoids. These are aromatic compounds containing a curcumin moiety, which is composed of two aryl buten-2-one (feruloyl) chromophores joined by a methylene group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Phenylpropanoids and polyketides |
|---|
| Class | Diarylheptanoids |
|---|
| Sub Class | Linear diarylheptanoids |
|---|
| Direct Parent | Curcuminoids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Curcumin
- Gingerdione
- Methoxyphenol
- Anisole
- Phenoxy compound
- Phenol ether
- Methoxybenzene
- Phenol
- 1-hydroxy-2-unsubstituted benzenoid
- Alkyl aryl ether
- 1,3-diketone
- Benzenoid
- 1,3-dicarbonyl compound
- Monocyclic benzene moiety
- Ketone
- Ether
- Organooxygen compound
- Organic oxygen compound
- Carbonyl group
- Hydrocarbon derivative
- Organic oxide
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | 0 |
|---|
| Melting point: | 95 - 97 C |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-004r-0910000000-fa5f9c88947d04535eb3 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0umr-9410140000-19c50b651dca5f38ec3c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0209000000-7dfd4c091aad667f4f26 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00bi-0922000000-4172923a1d3dfd5cefd5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004r-1911000000-8ecd77e0bdc5eeb2914c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-0109000000-cc999937febfc38f47c1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00di-0639000000-3347b9d7105791a849f6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-05ru-5986000000-5bfd0dc38ca2bb0b9efe | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1049000000-140fb262cdbb389db98d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00ks-6962000000-30302cd971363baba315 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-000i-3930000000-7ddc098840a945474666 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-00di-0219000000-9f478fd39c93a8ba2530 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-0935000000-5253debe0acf5be8a731 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-000i-1921000000-1003e2c392e655b3af0f | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Hassaninasab, A., Hashimoto, Y., Tomita-Yokotani, K., Kobayashi, M. (2011). "Discovery of the curcumin metabolic pathway involving a unique enzyme in an intestinal microorganism." Proc Natl Acad Sci U S A 108:6615-6620. Pubmed: 21467222
- Heath DD, Pruitt MA, Brenner DE, Begum AN, Frautschy SA, Rock CL: Tetrahydrocurcumin in plasma and urine: quantitation by high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2005 Sep 25;824(1-2):206-12. Pubmed: 16061427
|
|---|
| Synthesis Reference: | Lampe, W.; Smolinska, J. Ability of the two methyl groups of the quaternary base of 3,5-dimethylisoxazole to couple. III. Bull. acad. polon. sci., Ser. sci. chim., geol. et geograph (1958), 6 481-6. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 67263 | | HMDB ID | HMDB05789 | | Pubchem Compound ID | 124072 | | Kegg ID | Not Available | | ChemSpider ID | 110569 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|