| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:18:13 -0600 |
|---|
| Update Date | 2015-06-03 17:26:00 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
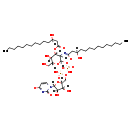
| Name: | UDP-2,3-bis(O-(3R)-3-Hydroxymyristoyl)-alpha-D-glucosamine |
|---|
| Description | UDP-2,3-bis(O-(3R)-3-Hydroxymyristoyl)-alpha-D-glucosamine is an intermediate in Lipid IVA synthesis. It is a substrate for the enzyme UDP-2,3-diacylglucosamine diphosphatase which catalyzes the reaction UDP-2,3-bis[(3R)-3-hydroxymyristoyl]-alpha-D-glucosamine + H2O = 2,3-bis[(3R)-3-hydroxymyristoyl]-beta-D-glucosaminyl 1-phosphate + UMP. |
|---|
| Structure | |
|---|
| Synonyms: | - OH-MYRISTOYL
- UDP-2,3-bis(3-hydroxymyristoyl)glucosamine
- UDP-2,3-bis(3-hydroxytetradecanoyl)glucosamine
- UDP-2,3-Bis(3R)-3-hydroxymyristoyl-a-D-glucosamine
- UDP-2,3-bis(3R)-3-hydroxymyristoyl-alpha-D-glucosamine
- UDP-2,3-Bis(3R)-3-hydroxymyristoyl-α-D-glucosamine
- UDP-2,3-Bis(O-(3R)-3-hydroxymyristoyl)-a-D-glucosamine
- UDP-2,3-Bis(O-(3R)-3-hydroxymyristoyl)-α-D-glucosamine
- UDP-2,3-biso-(3R)-3-Hydroxymyristoyl-a-D-glucosamine
- UDP-2,3-bisO-(3R)-3-hydroxymyristoyl-alpha-D-glucosamine
- UDP-2,3-biso-(3R)-3-Hydroxymyristoyl-α-D-glucosamine
- UDP-2,3-diacyl-glucosamine
|
|---|
| Chemical Formula: | C43H75N3O20P2 |
|---|
| Weight: | Average: 1016.0112
Monoisotopic: 1015.441914879 |
|---|
| InChI Key: | KOJCFMYSTWNMQW-RUAJDYCTSA-L |
|---|
| InChI: | InChI=1S/C43H77N3O20P2/c1-3-5-7-9-11-13-15-17-19-21-29(48)25-34(51)44-36-40(64-35(52)26-30(49)22-20-18-16-14-12-10-8-6-4-2)38(54)31(27-47)63-42(36)65-68(59,60)66-67(57,58)61-28-32-37(53)39(55)41(62-32)46-24-23-33(50)45-43(46)56/h23-24,29-32,36-42,47-49,53-55H,3-22,25-28H2,1-2H3,(H,44,51)(H,57,58)(H,59,60)(H,45,50,56)/p-2/t29-,30-,31-,32-,36-,37-,38-,39-,40-,41-,42-/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (3R)-N-[(2R,3R,4R,5S,6R)-2-({[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-oxido-2-oxo-1,2-dihydropyrimidin-1-yl)oxolan-2-yl]methoxy}(hydroxy)phosphoryl)oxy](hydroxy)phosphoryl}oxy)-5-hydroxy-6-(hydroxymethyl)-4-{[(3R)-3-hydroxytetradecanoyl]oxy}oxan-3-yl]-3-hydroxytetradecanecarboximidate |
|---|
| Traditional IUPAC Name: | (3R)-N-[(2R,3R,4R,5S,6R)-2-[({[(2R,3S,4R,5R)-3,4-dihydroxy-5-(4-oxido-2-oxopyrimidin-1-yl)oxolan-2-yl]methoxy(hydroxy)phosphoryl}oxy(hydroxy)phosphoryl)oxy]-5-hydroxy-6-(hydroxymethyl)-4-{[(3R)-3-hydroxytetradecanoyl]oxy}oxan-3-yl]-3-hydroxytetradecanecarboximidate |
|---|
| SMILES: | [H][C@@](O)(CCCCCCCCCCC)CC(=O)O[C@@]1([H])[C@]([H])(O)[C@@]([H])(CO)O[C@]([H])(OP(O)(=O)OP(O)(=O)OC[C@@]2([H])O[C@@]([H])(N3C=CC([O-])=NC3=O)[C@]([H])(O)[C@]2([H])O)[C@]1([H])N=C([O-])C[C@]([H])(O)CCCCCCCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pyrimidine nucleotide sugars. These are pyrimidine nucleotides bound to a saccharide derivative through the terminal phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Nucleosides, nucleotides, and analogues |
|---|
| Class | Pyrimidine nucleotides |
|---|
| Sub Class | Pyrimidine nucleotide sugars |
|---|
| Direct Parent | Pyrimidine nucleotide sugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pyrimidine nucleotide sugar
- Pyrimidine ribonucleoside diphosphate
- Acylaminosugar
- Saccharolipid
- Pentose phosphate
- Pentose-5-phosphate
- N-acyl-alpha-hexosamine
- Glycosyl compound
- N-glycosyl compound
- Monosaccharide phosphate
- Organic pyrophosphate
- Beta-hydroxy acid
- Fatty acid ester
- Pyrimidone
- Fatty amide
- Hydropyrimidine
- Hydroxy acid
- Monosaccharide
- N-acyl-amine
- Organic phosphoric acid derivative
- Oxane
- Fatty acyl
- Phosphoric acid ester
- Pyrimidine
- Alkyl phosphate
- Tetrahydrofuran
- Vinylogous amide
- Heteroaromatic compound
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxylic acid ester
- Urea
- Lactam
- Carboxamide group
- Monocarboxylic acid or derivatives
- Organoheterocyclic compound
- Oxacycle
- Carboxylic acid derivative
- Azacycle
- Organooxygen compound
- Organic oxide
- Organic nitrogen compound
- Alcohol
- Hydrocarbon derivative
- Organopnictogen compound
- Primary alcohol
- Organic oxygen compound
- Carbonyl group
- Organonitrogen compound
- Organic anion
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 78847 | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|