| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:17:43 -0600 |
|---|
| Update Date | 2015-06-03 17:25:59 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
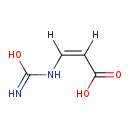
| Name: | (Z)-3-Ureidoacrylate |
|---|
| Description | (Z)-3-Ureidoacrylate is an intermediate in the degradation of pyrimidines. It is a substrate for the enzyme Peroxyureidoacrylate/ureidoacrylate amidohydrolase which catalyzes the reaction (Z)-3-ureidoacrylate peracid + H(2)O = (Z)-3-peroxyaminoacrylate + NH(3). This enzyme quickly hydrolyzes the ureidoacrylate peracid to avoid toxicity, but can also hydrolyzes ureidoacrylate that is formed spontaneously from ureidoacrylate peracid. |
|---|
| Structure | |
|---|
| Synonyms: | - (2Z)-3-(carbamoylamino)Acrylate
- (2Z)-3-(Carbamoylamino)acrylic acid
- (Z)-3-(carbamoylamino)Prop-2-enoate
- (Z)-3-(carbamoylamino)prop-2-enoic acid
- (Z)-3-ureido-2-Propenoate
- (Z)-3-ureido-2-propenoic acid
- (Z)-3-Ureidoacrylic acid
- Ureidoacrylate
- Ureidoacrylic acid
|
|---|
| Chemical Formula: | C4H6N2O3 |
|---|
| Weight: | Average: 130.102
Monoisotopic: 130.037842068 |
|---|
| InChI Key: | JDSSVQWHYUVDDF-UPHRSURJSA-N |
|---|
| InChI: | InChI=1S/C4H6N2O3/c5-4(9)6-2-1-3(7)8/h1-2H,(H,7,8)(H3,5,6,9)/b2-1- |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2Z)-3-[(C-hydroxycarbonimidoyl)amino]prop-2-enoic acid |
|---|
| Traditional IUPAC Name: | (2Z)-3-(C-hydroxycarbonimidoylamino)prop-2-enoic acid |
|---|
| SMILES: | [H]\C(NC(O)=N)=C(/[H])C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acrylic acids and derivatives. These are organic compounds containing acrylic acid CH2=CHCO2H or a derivative thereof. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Acrylic acids and derivatives |
|---|
| Direct Parent | Acrylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Acrylic acid or derivatives
- Vinylogous amide
- Urea
- Carbonic acid derivative
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 59890 | | HMDB ID | Not Available | | Pubchem Compound ID | 1751484 | | Kegg ID | C20254 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|