| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:17:30 -0600 |
|---|
| Update Date | 2015-06-03 17:25:58 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | L-3,4-Dihydroxybutan-2-one 4-phosphate |
|---|
| Description | L-3,4-Dihydroxybutan-2-one 4-phosphate is an intermediate involved in riboflavin metabolism. It is a substrate for the enzyme 6,7-dimethyl-8-ribityllumazine synthase which atalyzes the formation of 6,7-dimethyl-8-ribityllumazine by condensation of 5-amino-6-(D-ribitylamino)uracil with 3,4-dihydroxy-2-butanone 4-phosphate. This is the penultimate step in the biosynthesis of riboflavin. |
|---|
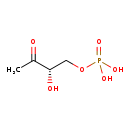
| Structure | |
|---|
| Synonyms: | - (2S)-2-hydroxy-3-oxobutyl dihydrogen phosphate
- (2S)-2-Hydroxy-3-oxobutyl dihydrogen phosphoric acid
- (3S)-3-hydroxy-4-(phosphonooxy)butan-2-one
- (S)-3-hydroxy-4-(phosphonooxy)-2-butanone
- 1-deoxy-L-glycero-tetrulose 4-phosphate
- 1-Deoxy-L-glycero-tetrulose 4-phosphoric acid
- 2-Butanone, 3-hydroxy-4-(phosphonooxy)-, (S)-
- 3,4-Dhbp
- 3,4-Dihydroxy-2-butanone-4-phosphate
- 3,4-Dihydroxy-2-butanone-4-phosphoric acid
- L-3,4-dihydroxybutan-2-one 4-phosphate
- L-3,4-Dihydroxybutan-2-one 4-phosphoric acid
|
|---|
| Chemical Formula: | C4H9O6P |
|---|
| Weight: | Average: 184.0844
Monoisotopic: 184.013674532 |
|---|
| InChI Key: | OKYHYXLCTGGOLM-BYPYZUCNSA-N |
|---|
| InChI: | InChI=1S/C4H9O6P/c1-3(5)4(6)2-10-11(7,8)9/h4,6H,2H2,1H3,(H2,7,8,9)/t4-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | [(2S)-2-hydroxy-3-oxobutoxy]phosphonic acid |
|---|
| Traditional IUPAC Name: | (2S)-2-hydroxy-3-oxobutoxyphosphonic acid |
|---|
| SMILES: | CC(=O)[C@@H](O)COP(O)(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as monoalkyl phosphates. These are organic compounds containing a phosphate group that is linked to exactly one alkyl chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Organic phosphoric acids and derivatives |
|---|
| Sub Class | Phosphate esters |
|---|
| Direct Parent | Monoalkyl phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Monoalkyl phosphate
- Monosaccharide
- Acyloin
- Alpha-hydroxy ketone
- Secondary alcohol
- Ketone
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Carbonyl group
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 50608 | | HMDB ID | Not Available | | Pubchem Compound ID | 14056322 | | Kegg ID | C15556 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|