| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:17:18 -0600 |
|---|
| Update Date | 2015-06-03 17:25:57 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
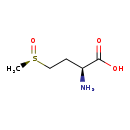
| Name: | L-Methionine (R)-S-oxide |
|---|
| Description | L-Methionine (R)-S-oxide or Met-(R)-O is an oxidized form of methionine. It can be used as a substrate for growth by E. coli. It is known that Escherichia coli methionine mutants can grow on both enantiomers of methionine sulfoxide (met(o)), i.e., Met-R-(O) or Met-S-(O), indicating the presence of enzymes in E. coli that can reduce each of these enantiomers to methionine (met). Recently it was discovered that an enzyme known as fRMsr or L-methionine (R)-S-oxide reductase (EC 1.8.4.14) is the enzyme that catalyzes the chemical reaction L-methionine + thioredoxin disulfide + H2O <=> L-methionine (R)-S-oxide + thioredoxin. It is thought that Met-(R)-O may represent a signaling molecule in response to oxidative stress. [PMID:17535911] |
|---|
| Structure | |
|---|
| Synonyms: | - (2S)-2-amino-4-(R)-Methylsulfinylbutanoate
- (2S)-2-Amino-4-(R)-methylsulfinylbutanoic acid
- (2S)-2-amino-4-(R)-Methylsulphinylbutanoate
- (2S)-2-amino-4-(R)-Methylsulphinylbutanoic acid
|
|---|
| Chemical Formula: | C5H11NO3S |
|---|
| Weight: | Average: 165.211
Monoisotopic: 165.045963913 |
|---|
| InChI Key: | QEFRNWWLZKMPFJ-ZXPFJRLXSA-N |
|---|
| InChI: | InChI=1S/C5H11NO3S/c1-10(9)3-2-4(6)5(7)8/h4H,2-3,6H2,1H3,(H,7,8)/t4-,10+/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2S)-2-amino-4-[(R)-methanesulfinyl]butanoic acid |
|---|
| Traditional IUPAC Name: | L-methionine (R)-S-oxide |
|---|
| SMILES: | C[S@@](=O)CC[C@H](N)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as l-alpha-amino acids. These are alpha amino acids which have the L-configuration of the alpha-carbon atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | L-alpha-amino acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - L-alpha-amino acid
- Thia fatty acid
- Fatty acid
- Fatty acyl
- Sulfoxide
- Amino acid
- Carboxylic acid
- Monocarboxylic acid or derivatives
- Sulfinyl compound
- Carbonyl group
- Organic oxygen compound
- Organic nitrogen compound
- Primary amine
- Organosulfur compound
- Organooxygen compound
- Organonitrogen compound
- Amine
- Primary aliphatic amine
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 49032 | | HMDB ID | Not Available | | Pubchem Compound ID | 10062737 | | Kegg ID | C15998 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available | | Ligand Expo | SME |
|
|---|