| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:17:09 -0600 |
|---|
| Update Date | 2015-06-03 17:25:57 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
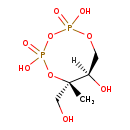
| Name: | 2-C-Methyl-D-erythritol 2,4-cyclodiphosphate |
|---|
| Description | 2-C-Methyl-D-erythritol 2,4-cyclodiphosphate or MECDP is a highly unusual cyclodiphosphate-containing intermediate in the mevalonate-independent pathway to isopentenyl diphosphate and dimethylallyl diphosphate (i.e. isoprenoid biosynthesis). It is a product of the enzyme 2-C-methyl-D-erythritol 2,4-cyclodiphosphate (MECDP) synthase which catalyzes the conversion of 4-diphosphocytidyl-2-C-methyl-D-erythritol 2-phosphate (CDP-ME2P) to MECDP [PMID: 11786530] |
|---|
| Structure | |
|---|
| Synonyms: | - (6S,7R)-2,4-dihydroxy-6-(hydroxymethyl)-6-methyl-2,4-dioxo-1,3,5,2$l^{5},4$l^{5}-trioxadiphosphocan-7-ol
- (6S,7R)-6-(Hydroxymethyl)-6-methyl-1,3,5,2,4-trioxadiphosphocane-2,4,7-triol 2,4-dioxide
- 2-C-Methyl-D-erythritol 2,4-cyclodiphosphoric acid
- 3-Methyl-1,2,3,4-tetrahydroxybutane-1,3-cyclic bisphosphate
- 3-Methyl-1,2,3,4-tetrahydroxybutane-1,3-cyclic bisphosphoric acid
|
|---|
| Chemical Formula: | C5H12O9P2 |
|---|
| Weight: | Average: 278.0909
Monoisotopic: 277.995655006 |
|---|
| InChI Key: | SFRQRNJMIIUYDI-UHNVWZDZSA-N |
|---|
| InChI: | InChI=1S/C5H12O9P2/c1-5(3-6)4(7)2-12-15(8,9)14-16(10,11)13-5/h4,6-7H,2-3H2,1H3,(H,8,9)(H,10,11)/t4-,5+/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (6S,7R)-2,4,7-trihydroxy-6-(hydroxymethyl)-6-methyl-1,3,5,2λ⁵,4λ⁵-trioxadiphosphocane-2,4-dione |
|---|
| Traditional IUPAC Name: | 3-mthbcp |
|---|
| SMILES: | [H][C@@]1(O)COP(O)(=O)OP(O)(=O)O[C@@]1(C)CO |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as organic pyrophosphates. These are organic compounds containing the pyrophosphate oxoanion, with the structure OP([O-])(=O)OP(O)([O-])=O. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organic oxoanionic compounds |
|---|
| Sub Class | Organic pyrophosphates |
|---|
| Direct Parent | Organic pyrophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic pyrophosphate
- Organic phosphoric acid derivative
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|