| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:16:42 -0600 |
|---|
| Update Date | 2015-06-03 17:25:55 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
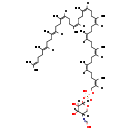
| Name: | 4-Amino-4-deoxy-alpha-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphate |
|---|
| Description | 4-Amino-4-deoxy-alpha-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphate is an intermediate in LPS synthesis. It is a substrate for Undecaprenyl-phosphate 4-deoxy-4-formamido-L-arabinose transferase which catalyzes the reaction UDP-4-deoxy-4-formamido-beta-L-arabinose + di-trans,octa-cis-undecaprenyl phosphate = UDP + 4-deoxy-4-formamido-alpha-L-arabinose di-trans,octa-cis-undecaprenyl phosphate. |
|---|
| Structure | |
|---|
| Synonyms: | - 4-amino-4-Deoxy-a-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphate
- 4-amino-4-Deoxy-a-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphoric acid
- 4-amino-4-Deoxy-alpha-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphoric acid
- 4-amino-4-Deoxy-α-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphate
- 4-amino-4-Deoxy-α-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphoric acid
- UDP-Ara4FN
|
|---|
| Chemical Formula: | C61H100NO8P |
|---|
| Weight: | Average: 1006.4224
Monoisotopic: 1005.718655693 |
|---|
| InChI Key: | KDTATMYQJZYGGT-CRHUQJHVSA-N |
|---|
| InChI: | InChI=1S/C61H100NO8P/c1-47(2)23-13-24-48(3)25-14-26-49(4)27-15-28-50(5)29-16-30-51(6)31-17-32-52(7)33-18-34-53(8)35-19-36-54(9)37-20-38-55(10)39-21-40-56(11)41-22-42-57(12)43-44-69-71(66,67)70-61-60(65)59(64)58(45-68-61)62-46-63/h23,25,27,29,31,33,35,37,39,41,43,46,58-61,64-65H,13-22,24,26,28,30,32,34,36,38,40,42,44-45H2,1-12H3,(H,62,63)(H,66,67)/b48-25+,49-27+,50-29-,51-31-,52-33-,53-35-,54-37-,55-39-,56-41-,57-43-/t58-,59-,60+,61-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | N-[(3S,4S,5R,6S)-4,5-dihydroxy-6-{[hydroxy({[(2Z,6Z,10Z,14Z,18Z,22Z,26Z,30Z,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl]oxy})phosphoryl]oxy}oxan-3-yl]carboximidic acid |
|---|
| Traditional IUPAC Name: | N-[(3S,4S,5R,6S)-4,5-dihydroxy-6-({hydroxy[(2Z,6Z,10Z,14Z,18Z,22Z,26Z,30Z,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl]oxyphosphoryl}oxy)oxan-3-yl]carboximidic acid |
|---|
| SMILES: | OC=N[C@@]1([H])CO[C@@]([H])(OP(O)(=O)OC\C([H])=C(\C)CC\C([H])=C(\C)CC\C([H])=C(\C)CC\C([H])=C(\C)CC\C([H])=C(\C)CC\C([H])=C(\C)CC\C([H])=C(\C)CC\C([H])=C(\C)CC\C([H])=C(/C)CC\C([H])=C(/C)CCC=C(C)C)[C@@](O)([H])[C@]1(O)[H] |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as bactoprenol monophosphates. These are polyprenyl compounds consisting of a monophosphate group substituted by a bactoprenyl moiety. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Polyprenols |
|---|
| Direct Parent | Bactoprenol monophosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polyterpenoid
- Bactoprenol monophosphate
- Polyprenyl monophosphate
- Isoprenoid phosphate
- Dialkyl phosphate
- Monosaccharide
- Organic phosphoric acid derivative
- Oxane
- Phosphoric acid ester
- Alkyl phosphate
- Carboxamide group
- Secondary carboxylic acid amide
- Secondary alcohol
- 1,2-diol
- Oxacycle
- Organoheterocyclic compound
- Carboxylic acid derivative
- Hydrocarbon derivative
- Organonitrogen compound
- Alcohol
- Organic oxide
- Organooxygen compound
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | Uridine 5''-diphospho-{beta}-4-deoxy-4-formamido-L-arabinose + Di-trans,poly-cis-undecaprenyl phosphate <> Undecaprenyl phosphate alpha-L-Ara4FN + Uridine 5'-diphosphate + 4-Amino-4-deoxy-alpha-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphate4-Amino-4-deoxy-alpha-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphate + 2,3,2'3'-Tetrakis(beta-hydroxymyristoyl)-D-glucosaminyl-1,6-beta-D-glucosamine 1,4'-bisphosphate > lipid II(A) + di-trans,octa-cis-undecaprenyl phosphate2 4-Amino-4-deoxy-alpha-L-arabinopyranosyl di-trans,octa-cis-undecaprenyl phosphate + (KDO)2-lipid A >2 di-trans,octa-cis-undecaprenyl diphosphate + L-Ara4N-modified KDO2-Lipid A |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | - Amino sugar and nucleotide sugar metabolism ec00520
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 53028 | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | C16156 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|