| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-10-10 12:15:02 -0600 |
|---|
| Update Date | 2015-06-03 17:25:52 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
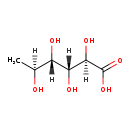
| Name: | L-Rhamnonate |
|---|
| Description | L-Rhamnoate (6-deoxy-L-mannonate) is a mono-acid sugar. It is the preferred substrate for the E. coli enzyme called L-Rhamnoate dehydratase (YfaW). L-Rhamnonate, L-mannonate, and L-lyxonate share the same configurations at carbons-2, -3, and -4; D-gulonate differs from L-rhamnonate and L-mannonate by the configuration of carbon-5. |
|---|
| Structure | |
|---|
| Synonyms: | - 6-Deoxy-L-mannonate
- 6-Deoxy-L-mannonic acid
- L-Rhamnonic acid
|
|---|
| Chemical Formula: | C6H12O6 |
|---|
| Weight: | Average: 180.1559
Monoisotopic: 180.063388116 |
|---|
| InChI Key: | NBFWIISVIFCMDK-QMKXCQHVSA-N |
|---|
| InChI: | InChI=1S/C6H12O6/c1-2(7)3(8)4(9)5(10)6(11)12/h2-5,7-10H,1H3,(H,11,12)/t2-,3-,4+,5+/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2R,3R,4S,5S)-2,3,4,5-tetrahydroxyhexanoic acid |
|---|
| Traditional IUPAC Name: | L-rhamnonic acid |
|---|
| SMILES: | [H][C@@](C)(O)[C@]([H])(O)[C@@]([H])(O)[C@@]([H])(O)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as medium-chain hydroxy acids and derivatives. These are hydroxy acids with a 6 to 12 carbon atoms long side chain. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Hydroxy acids and derivatives |
|---|
| Sub Class | Medium-chain hydroxy acids and derivatives |
|---|
| Direct Parent | Medium-chain hydroxy acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Medium-chain hydroxy acid
- Medium-chain fatty acid
- Beta-hydroxy acid
- Hydroxy fatty acid
- Alpha-hydroxy acid
- Monosaccharide
- Fatty acyl
- Fatty acid
- Secondary alcohol
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Polyol
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Carbonyl group
- Alcohol
- Organic oxygen compound
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | - Fructose and mannose metabolism ec00051
- Microbial metabolism in diverse environments ec01120
|
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 17357 | | HMDB ID | Not Available | | Pubchem Compound ID | 6602429 | | Kegg ID | C01934 | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|