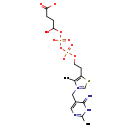| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-08-09 09:25:32 -0600 |
|---|
| Update Date | 2015-06-03 17:21:48 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Succinate-semialdehyde-thiamine PPi |
|---|
| Description | Succinate-semialdehyde-thiamine pyrophosphate is a thiamine derivative. The first intermediate (SHCHC) in the menaquinone biosynthetic pathway, requires two reactions. The first is the decarboxylation of alpha-ketoglutarate by an alpha-ketoglutarate decarboxylase, which results in the formation of succinic semialdehyde-thiamine PPi (TPP) anion, and the second is the addition of the succinic semialdehyde-TPP anion to isochorismate, which is carried out by the enzyme SHCHC synthase. Both enzymatic activities are encoded by the menD gene [PMID: 1459959] |
|---|
| Structure | |
|---|
| Synonyms: | - Succinic acid-semialdehyde-thiamine ppi
- Succinic semialdehyde-thiamine pyrophosphate
- Succinic semialdehyde-thiamine pyrophosphoric acid
|
|---|
| Chemical Formula: | C16H22N4O10P2S |
|---|
| Weight: | Average: 524.379
Monoisotopic: 524.053186658 |
|---|
| InChI Key: | JUWXHVVKSHTJQQ-UHFFFAOYSA-L |
|---|
| InChI: | InChI=1S/C16H24N4O10P2S/c1-10-13(33-9-20(10)8-12-7-18-11(2)19-16(12)17)5-6-28-31(24,25)30-32(26,27)29-15(23)4-3-14(21)22/h7,9,15,23H,3-6,8H2,1-2H3,(H4-,17,18,19,21,22,24,25,26,27)/p-2 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 5-[2-({[(3-carboxylato-1-hydroxypropyl phosphonato)oxy]phosphinato}oxy)ethyl]-3-[(6-imino-2-methyl-1,6-dihydropyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium |
|---|
| Traditional IUPAC Name: | 5-(2-{[(3-carboxylato-1-hydroxypropyl phosphonato)oxyphosphinato]oxy}ethyl)-3-[(4-imino-2-methyl-3H-pyrimidin-5-yl)methyl]-4-methyl-1,3-thiazol-3-ium |
|---|
| SMILES: | CC1=C(CCOP([O-])(=O)OP([O-])(=O)OC(O)CCC([O-])=O)SC=[N+]1CC1=CN=C(C)NC1=N |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thiamine phosphates. These are thiamine derivatives in which the hydroxyl group of the ethanol moiety is substituted by a phosphate group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Diazines |
|---|
| Sub Class | Pyrimidines and pyrimidine derivatives |
|---|
| Direct Parent | Thiamine phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Thiamine-phosphate
- Organic pyrophosphate
- 4,5-disubstituted 1,3-thiazole
- Imidolactam
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- Hydropyrimidine
- Heteroaromatic compound
- Thiazole
- Azole
- Carboxylic acid salt
- Azacycle
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organic salt
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Organic anion
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -2 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 45479398 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|