| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-08-09 09:25:06 -0600 |
|---|
| Update Date | 2015-06-03 17:21:37 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
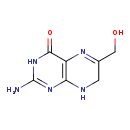
| Name: | 2-Amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine |
|---|
| Description | 2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine is a member of the chemical class known as Pterins and Derivatives. These are polycyclic aromatic compounds containing a pterin moeity, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one. 2-amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine is invovled in Folate biosynthesis. (KEGG) |
|---|
| Structure | |
|---|
| Synonyms: | - 2-Amino-4-hydroxy-6-hydroxymethyl-7,8-dihydropteridine
- 2-Amino-6-(hydroxymethyl)-7,8-dihydropteridin-4-ol
- HMeDHP
|
|---|
| Chemical Formula: | C7H9N5O2 |
|---|
| Weight: | Average: 195.1787
Monoisotopic: 195.075624557 |
|---|
| InChI Key: | CQQNNQTXUGLUEV-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C7H9N5O2/c8-7-11-5-4(6(14)12-7)10-3(2-13)1-9-5/h13H,1-2H2,(H4,8,9,11,12,14) |
|---|
| CAS number: | 3672-03-5 |
|---|
| IUPAC Name: | 2-amino-6-(hydroxymethyl)-3,4,7,8-tetrahydropteridin-4-one |
|---|
| Traditional IUPAC Name: | 2-amino-6-(hydroxymethyl)-7,8-dihydro-3H-pteridin-4-one |
|---|
| SMILES: | NC1=NC2=C(N=C(CO)CN2)C(=O)N1 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pterins and derivatives. These are polycyclic aromatic compounds containing a pterin moiety, which consist of a pteridine ring bearing a ketone and an amine group to form 2-aminopteridin-4(3H)-one. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Pterins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pterin
- Secondary aliphatic/aromatic amine
- Hydroxypyrimidine
- Pyrimidine
- Heteroaromatic compound
- Ketimine
- Azacycle
- Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Secondary amine
- Alcohol
- Organopnictogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Imine
- Amine
- Hydrocarbon derivative
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-02t9-1900000000-5cfea5c037994f006216 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-002b-0900000000-9f8713d042cc448282c0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-0900000000-443afedc044efb6836ef | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01p9-2900000000-143274abf0f8afadfb00 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-7d20bbe8055a463789f9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01r6-1900000000-56b39cb20c728828c72a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9200000000-32e7a15f26d685ec66bc | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0002-0900000000-e6264bd1af5f7597a2c4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0002-0900000000-2046c8dbdea1405f54d1 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004l-4900000000-8df0786ac08c282820b0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0006-0900000000-d9a36ecaf7ce4795a113 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-1900000000-695ce544726c989be876 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9400000000-441d6e22f231bf631645 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 17083 | | HMDB ID | HMDB0304227 | | Pubchem Compound ID | 218 | | Kegg ID | C01300 | | ChemSpider ID | 213 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available | | Ligand Expo | PH2 |
|
|---|