| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-08-09 09:16:18 -0600 |
|---|
| Update Date | 2015-09-13 12:56:17 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
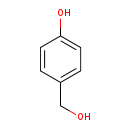
| Name: | 4-Hydroxybenzyl alcohol |
|---|
| Description | 4-hydroxybenzyl alcohol is the cleavage product produced during the biosynthesis of the thiazole moiety of thiamine from tyrosine as part of the thiamine biosynthesis pathway. (PMID 369616, EcoCyc) |
|---|
| Structure | |
|---|
| Synonyms: | - 4-(Hydroxymethyl)phenol
- 4-(hydroxymethyl)phenol (ACD/Name 4.0)
- 4-hydroxybenzenemethanol
- 4-Hydroxybenzylalcohol
- 4-Methylolphenol
- 4-Methylophenol
- A-hydroxy-p-cresol
- Alpha-hydroxy-p-cresol
- B4-hydroxy-enzenemethanol
- Gastrodigenin
- P-(hydroxymethyl)phenol
- P-hydroxy-Benzyl alcohol
- P-Hydroxybenzyl alcohol
- P-Methylolphenol
- Para-Hydroxybenzyl alcohol
- α-Hydroxy-P-cresol
|
|---|
| Chemical Formula: | C7H8O2 |
|---|
| Weight: | Average: 124.1372
Monoisotopic: 124.0524295 |
|---|
| InChI Key: | BVJSUAQZOZWCKN-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C7H8O2/c8-5-6-1-3-7(9)4-2-6/h1-4,8-9H,5H2 |
|---|
| CAS number: | 623-05-2 |
|---|
| IUPAC Name: | 4-(hydroxymethyl)phenol |
|---|
| Traditional IUPAC Name: | P-hydroxybenzyl alcohol |
|---|
| SMILES: | OCC1=CC=C(O)C=C1 |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as benzyl alcohols. These are organic compounds containing the phenylmethanol substructure. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzyl alcohols |
|---|
| Direct Parent | Benzyl alcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Benzyl alcohol
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Organic oxygen compound
- Hydrocarbon derivative
- Aromatic alcohol
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | 0 |
|---|
| Melting point: | 124.5 °C |
|---|
| Experimental Properties: | | Property | Value | Source |
|---|
| LogP: | 0.25 [HANSCH,C ET AL. (1995)] | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-05i1-9400000000-f08047b4457dc23151d9 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0gdi-0970000000-2415f3f14aceb1b2477b | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00os-1930000000-4cb3e427ccd00fb05cef | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-004j-1930000000-8da8f2d1a4cd6945c0d2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00os-0930000000-fb426d5a8be1daafc38c | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-9830000000-37513a80b983842a093d | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-05i1-9400000000-f08047b4457dc23151d9 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-0gdi-0970000000-2415f3f14aceb1b2477b | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00os-1930000000-4cb3e427ccd00fb05cef | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-004j-1930000000-8da8f2d1a4cd6945c0d2 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00os-0930000000-fb426d5a8be1daafc38c | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-00di-9830000000-37513a80b983842a093d | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-05fv-9700000000-75343ed1558a8215cd86 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-0fmi-8930000000-dc7e6a9414fca90c09ff | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-0900000000-e86139fcdae517d0dd50 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0a4i-1900000000-23d9f02bc9bfae1b0404 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a6r-9500000000-ef57e905020db4322452 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-00di-1900000000-08d6d0fac3014c21f4e4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-00dl-5900000000-a615d3a76cf31c5305cf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9100000000-e8119b2a0a6f66edc346 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-056r-1900000000-14e2a5a869f98931316e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9200000000-3f2037d44894ccf1af87 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-004i-9100000000-5896e65cef551a4d404f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-006x-9600000000-2e7ed49ef552bb91252d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9500000000-39119700dc572f82fda6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-ade1ec5c4dc45647bb63 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Nair B: Final report on the safety assessment of Benzyl Alcohol, Benzoic Acid, and Sodium Benzoate. Int J Toxicol. 2001;20 Suppl 3:23-50. Pubmed: 11766131
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- White, R. H. (1979). "4-Hydroxybenzyl alcohol. A metabolite produced during the biosynthesis of thiamine in Escherichia coli." Biochim Biophys Acta 583:55-62. Pubmed: 369616
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| Links |
|---|
| External Links: | |
|---|