| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-08-09 09:16:15 -0600 |
|---|
| Update Date | 2015-09-13 12:56:16 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Imidazolelactic acid |
|---|
| Description | Imidazolelactic acid is a compound that has an imidazole group attached to the beta carbon of lactic acid. (inferred from compound structure) Imidazolelactic acid can be formed from an NADH-assisted reduction of imidazol-5-yl-pyruvate. (KEGG) |
|---|
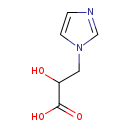
| Structure | |
|---|
| Synonyms: | - (S)-3-(Imidazol-5-yl)-lactate
- (S)-3-(Imidazol-5-yl)-lactic acid
- 1-Imidazolelactate
- 1-Imidazolelactic acid
- 2-Hydroxy-3-[4-imidazolyl]-propanoate
- 2-Hydroxy-3-[4-imidazolyl]-propanoic acid
- (S)-3-(imidazol-5-yl)-lactate
- Imidazol-5-yl-lactate
- Imidazol-5-yl-lactic acid
- Imidazole lactate
- Imidazole lactic acid
- Imidazolelactate
|
|---|
| Chemical Formula: | C6H8N2O3 |
|---|
| Weight: | Average: 156.1393
Monoisotopic: 156.053492132 |
|---|
| InChI Key: | JTYMXXCJQKGGFG-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C6H8N2O3/c9-5(6(10)11)3-8-2-1-7-4-8/h1-2,4-5,9H,3H2,(H,10,11) |
|---|
| CAS number: | 876-19-7 |
|---|
| IUPAC Name: | 2-hydroxy-3-(1H-imidazol-1-yl)propanoic acid |
|---|
| Traditional IUPAC Name: | 2-hydroxy-3-(imidazol-1-yl)propanoic acid |
|---|
| SMILES: | OC(CN1C=CN=C1)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as imidazolyl carboxylic acids and derivatives. These are organic compounds containing a carboxylic acid chain (of at least 2 carbon atoms) linked to an imidazole ring. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Azoles |
|---|
| Sub Class | Imidazoles |
|---|
| Direct Parent | Imidazolyl carboxylic acids and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Imidazolyl carboxylic acid derivative
- Alpha-hydroxy acid
- Hydroxy acid
- N-substituted imidazole
- Heteroaromatic compound
- Secondary alcohol
- Carboxylic acid derivative
- Carboxylic acid
- Azacycle
- Monocarboxylic acid or derivatives
- Alcohol
- Organonitrogen compound
- Organooxygen compound
- Hydrocarbon derivative
- Organic oxide
- Organopnictogen compound
- Organic oxygen compound
- Carbonyl group
- Organic nitrogen compound
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001i-9100000000-af6e1c31ba814d5c0085 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-001i-9440000000-51a0a80ace23d0a3d85e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-052r-0900000000-0329a4bafbb746648ef5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-000i-5900000000-2ceb5bb4c7d0c4d563ae | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-014i-9000000000-af66fc635cd0e0618e4b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0a4i-1900000000-e77f735cce68a7f7b4a2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9300000000-6a5f23eb647fb89baad5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014l-9000000000-4e4a86f260b34da6c02a | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0aor-9800000000-3a57c8d2b2b5cf3cf713 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-014i-9000000000-a2f57dd2d7faa84a9a49 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-014i-9000000000-281cf185205743f8c55b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0bt9-0900000000-81381fab6df16fa55b59 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-00lr-9400000000-eef5c8eb5c6f485c4b70 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0gb9-9000000000-a7ce72d207b1b75057fd | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Block WD, Westhoff MH, Steele BF: Histidine metabolism in the human adult: histidine blood tolerance, and the effect of continued free L-histidine ingestion on the concentration of imidazole compounds in blood and urine. J Nutr. 1967 Feb;91(2):189-94. Pubmed: 6021220
- Dubovsky J, Dubovska E, Formankova J: [Imidazolactic acid in the urine. Method of determination and clinical observations]. Cas Lek Cesk. 1965 Nov 5;104(44):1216-21. Pubmed: 5856262
- Hamacher M, Mosebach KO: [Quantitative determination of some imidazole derivatives in the urine of pregnant patients, non-pregnant patients and those with diseases caused by pregnancy]. Hoppe Seylers Z Physiol Chem. 1969 May;350(5):603-8. Pubmed: 5789877
- Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM: Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009 Feb 12;457(7231):910-4. Pubmed: 19212411
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- van Roon-Djordjevic B, Cerfontain-van Staalen: Urinary excretion of histidine metabolites as an indication for folic acid and vitamin B 12 deficiency. Clin Chim Acta. 1972 Oct;41:55-65. Pubmed: 4645251
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| Links |
|---|
| External Links: | |
|---|