| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-08-09 09:16:14 -0600 |
|---|
| Update Date | 2015-09-13 15:15:33 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Salicylic acid |
|---|
| Description | Salicylic acid is a colorless, crystalline organic carboxylic acid. Salicylic acid is toxic if ingested in large quantities, but in small quantities is used as a food preservative and antiseptic in toothpaste. It is also the key additive in many skin-care products for the treatment of acne, psoriasis, callouses, corns, keratosis pilaris and warts. The carboxyl group (COOH) can react with alcohols, forming several useful esters. The name derives from the latin word for the willow tree (Salix), from whose bark it can be obtained. Salicylate is a known inducer of the multiple antibiotic resistance protein MarA |
|---|
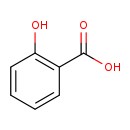
| Structure | |
|---|
| Synonyms: | - 2-Carboxyphenol
- 2-HBA
- 2-Hydroxybenzenecarboxylate
- 2-Hydroxybenzenecarboxylic acid
- 2-Hydroxybenzoate
- 2-Hydroxybenzoic acid
- Advanced Pain Relief Callus Removers
- Advanced Pain Relief Corn Removers
- Clear away Wart Remover
- Compound W
- Dr. Scholl's Callus Removers
- Dr. Scholl's Corn Removers
- Dr. Scholl's Wart Remover Kit
- Duofil Wart Remover
- Duoplant
- Freezone
- Ionil
- Ionil Plus
- K 537
- K 557
- O-Carboxyphenol
- O-Hydroxybenzoate
- O-Hydroxybenzoic acid
- Phenol-2-carboxylate
- Phenol-2-carboxylic acid
- Psoriacid-S-Stift
- Retarder W
- Rutranex
- SA
- Salicylate
- Salicylate collodion
- Salicylate soap
- Salicylic acid collodion
- Salicylic acid Soap
- Saligel
- Salonil
- Stri-Dex
- Trans-Ver-Sal
|
|---|
| Chemical Formula: | C7H6O3 |
|---|
| Weight: | Average: 138.1207
Monoisotopic: 138.031694058 |
|---|
| InChI Key: | YGSDEFSMJLZEOE-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C7H6O3/c8-6-4-2-1-3-5(6)7(9)10/h1-4,8H,(H,9,10) |
|---|
| CAS number: | 69-72-7 |
|---|
| IUPAC Name: | 2-hydroxybenzoic acid |
|---|
| Traditional IUPAC Name: | salicylic |
|---|
| SMILES: | OC(=O)C1=CC=CC=C1O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as salicylic acids. These are ortho-hydroxylated benzoic acids. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Benzenoids |
|---|
| Class | Benzene and substituted derivatives |
|---|
| Sub Class | Benzoic acids and derivatives |
|---|
| Direct Parent | Salicylic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Salicylic acid
- Benzoic acid
- Benzoyl
- 1-hydroxy-4-unsubstituted benzenoid
- 1-hydroxy-2-unsubstituted benzenoid
- Phenol
- Vinylogous acid
- Monocarboxylic acid or derivatives
- Carboxylic acid
- Carboxylic acid derivative
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Organooxygen compound
- Aromatic homomonocyclic compound
|
|---|
| Molecular Framework | Aromatic homomonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | -1 |
|---|
| Melting point: | 158 °C |
|---|
| Experimental Properties: | | Property | Value | Source |
|---|
| Water Solubility: | 2.24 mg/mL at 25 oC [YALKOWSKY,SH & DANNENFELSER,RM (1992)] | PhysProp | | LogP: | 2.26 [HANSCH,C ET AL. (1995)] | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | Collection of Reactions without pathways | PW001891 |    |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (2 TMS) | splash10-014i-3890000000-62eae168a9d7ab3ada6f | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-00du-9700000000-e1e2ee6b61d86c596403 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-014i-3890000000-62eae168a9d7ab3ada6f | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-EI-TOF (Non-derivatized) | splash10-014i-2960000000-1b6b46cbb2b643b71448 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-0079-8900000000-e8ee46d81fcc1ce3766e | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (2 TMS) - 70eV, Positive | splash10-006x-8950000000-9ed3a56f2b2654ba281f | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 10V, Negative (Annotated) | splash10-059j-9600000000-54545731fceee84be340 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 25V, Negative (Annotated) | splash10-00xu-9500000000-2f1c989b672669aaf083 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - Quattro_QQQ 40V, Negative (Annotated) | splash10-0gb9-9000000000-a0049e982e8ecd7ab730 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 10V, Negative | splash10-000i-0900000000-f1e71df6894bcc8dda74 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 20V, Negative | splash10-0006-9200000000-f9fd317c182ec7ca90dc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 30V, Negative | splash10-0006-9000000000-2b17aea4ee0ddd6321cf | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 40V, Negative | splash10-0006-9000000000-320b7cd879b61439cf42 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ (API3000, Applied Biosystems) 50V, Negative | splash10-0006-9000000000-7d1b96d60026076a7ecc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF , Negative | splash10-002b-0496100000-97708001d2a6d031beff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-002b-0496100000-97708001d2a6d031beff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF , Negative | splash10-002b-0496100000-97708001d2a6d031beff | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - ESI-TOF 10V, Negative | splash10-000i-0900000000-f88c693bac9b89416a52 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - DI-ESI-qTof , Positive | splash10-00di-0900000000-2aeace8112266d938c2b | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0006-9400000000-b0fb5458dfa73429b976 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0006-9400000000-b0fb5458dfa73429b976 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0006-9100000000-237ee14e8af5262c0dab | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0006-9000000000-3ec5d7a9114e37b8af2a | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0006-9000000000-d8fdab29114453b10280 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QFT , negative | splash10-0006-9000000000-4a337e3639c9f42a9000 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-2900000000-23d1cf43d4dedc979389 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0076-8900000000-f8b39b175209523386d0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0udi-9000000000-549ee40f4c3d2a965b2d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000l-6900000000-06bd3bf75f92d507fd8d | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0006-9100000000-fd107d170618784f2f1f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0006-9000000000-3fc7a3b941f5e3e4f7dd | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-00du-9600000000-6d4a0ff2d48d814b5c54 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H,1H] 2D NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 2D NMR | [1H,13C] 2D NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Alanko K, Stubb S, Salo OP, Reitamo S: Suction blister fluid histamine in fixed drug eruption. Acta Derm Venereol. 1992;72(2):89-91. Pubmed: 1350413
- Azaroual, Imbenotte M, Cartigny B, Lhermitte M, Vermeersch G: [Identification and quantification of exogenous metabolites in biological liquids with new development in NMR spectroscopy in one and two dimensions] Acta Clin Belg Suppl. 1999;1:97-100. Pubmed: 10216993
- Baggott JE, Morgan SL, Ha T, Vaughn WH, Hine RJ: Inhibition of folate-dependent enzymes by non-steroidal anti-inflammatory drugs. Biochem J. 1992 Feb 15;282 ( Pt 1):197-202. Pubmed: 1540135
- Benfeldt E, Serup J, Menne T: Microdialysis vs. suction blister technique for in vivo sampling of pharmacokinetics in the human dermis. Acta Derm Venereol. 1999 Sep;79(5):338-42. Pubmed: 10494706
- Berkovitch M, Uziel Y, Greenberg R, Chen-Levy Z, Arcusin M, Marcus O, Pinto O, Evans S, Matias A, Lahat E: False-high blood salicylate levels in neonates with hyperbilirubinemia. Ther Drug Monit. 2000 Dec;22(6):757-61. Pubmed: 11128247
- Flower R, Gryglewski R, Herbaczynska-Cedro K, Vane JR: Effects of anti-inflammatory drugs on prostaglandin biosynthesis. Nat New Biol. 1972 Jul 26;238(82):104-6. Pubmed: 4505422
- Goussis OS, Theodoropoulos TJ: Dilantin and salicylate effects on hepatic thyroxine bio-availability and dialyzable thyroxine. Horm Metab Res. 1990 Jun;22(6):342-4. Pubmed: 2379917
- Hazouard E, Grimbert M, Jonville-Berra AP, De Toffol MC, Legras A: [Salicylism and glaucoma: reciprocal augmentation of the toxicity of acetazolamide and acetylsalicylic acid] J Fr Ophtalmol. 1999 Feb;22(1):73-5. Pubmed: 10221197
- Khan AZ, Aarons L: A note on the use of salicylate saliva concentration in clinical pharmacokinetic studies. J Pharm Pharmacol. 1989 Oct;41(10):710-1. Pubmed: 2575150
- Kocoshis SA, Wong CT: Sodium salicylate and bile acid-induced colonic secretion in the rat. Ann Clin Lab Sci. 1991 May-Jun;21(3):197-204. Pubmed: 2064304
- Kunkel A, Watzig H: Pharmacokinetic investigations with direct injection of plasma samples: possible savings using capillary electrophoresis (CE). Arch Pharm (Weinheim). 1999 May;332(5):175-8. Pubmed: 10366903
- Ndovi TT, Choi L, Caffo B, Parsons T, Baker S, Zhao M, Rohde C, Hendrix CW: Quantitative assessment of seminal vesicle and prostate drug concentrations by use of a noninvasive method. Clin Pharmacol Ther. 2006 Aug;80(2):146-58. Pubmed: 16890576
- Owen SG, Francis HW, Roberts MS: Disappearance kinetics of solutes from synovial fluid after intra-articular injection. Br J Clin Pharmacol. 1994 Oct;38(4):349-55. Pubmed: 7833225
- Pirola R, Bareggi SR, De Benedittis G: Determination of acetylsalicylic acid and salicylic acid in skin and plasma by high-performance liquid chromatography. J Chromatogr B Biomed Sci Appl. 1998 Feb 13;705(2):309-15. Pubmed: 9521569
- Quaranta A, Portalatini P, Camporeale M, Sallustio V: Effects of salicylates on evoked otoacoustic emissions and remote masking in humans. Audiology. 1999 May-Jun;38(3):174-9. Pubmed: 10437688
- Rutner M, Fitzek J, Jahnel-Kracht H, Otto J, Krause W: [Therapy of rheumatic disease with a hydroxyethylsalicylate gel. Results of 2 clinical studies of effectiveness and bioavailability] Fortschr Med. 1995 Mar 20;113(8):111-3. Pubmed: 7759034
- Schmook FP, Meingassner JG, Billich A: Comparison of human skin or epidermis models with human and animal skin in in-vitro percutaneous absorption. Int J Pharm. 2001 Mar 14;215(1-2):51-6. Pubmed: 11250091
- Singh P, Anliker M, Smith GA, Zavortink D, Maibach HI: Transdermal iontophoresis and solute penetration across excised human skin. J Pharm Sci. 1995 Nov;84(11):1342-6. Pubmed: 8587053
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Vane JR: Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232-5. Pubmed: 5284360
- Vila MM, Tubino M, de Oliveira Neto G: Determination of salicylate in blood serum by flow injection with immobilized salicylate hydroxylase. J AOAC Int. 2001 Sep-Oct;84(5):1363-9. Pubmed: 11601455
- Yoshida NH, Roberts MS: Prediction of cathodal iontophoretic transport of various anions across excised skin from different vehicles using conductivity measurements. J Pharm Pharmacol. 1995 Nov;47(11):883-90. Pubmed: 8708980
- Zaugg S, Zhang X, Sweedler J, Thormann W: Determination of salicylate, gentisic acid and salicyluric acid in human urine by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr B Biomed Sci Appl. 2001 Mar 5;752(1):17-31. Pubmed: 11254191
|
|---|
| Synthesis Reference: | Yin, Yingwu; Guo, Qingbin. Preparation of salicylic acid from phenol. Faming Zhuanli Shenqing Gongkai Shuomingshu (2005), 7pp. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| Links |
|---|
| External Links: | |
|---|