| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-08-03 15:09:33 -0600 |
|---|
| Update Date | 2015-06-03 17:21:26 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
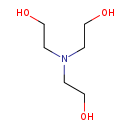
| Name: | Triethanolamine |
|---|
| Description | Triethanolamine, often abbreviated as TEA, is an organic compound that is both a tertiary amine and a triol. A triol is a molecule with three alcohol groups. Like other amines, triethanolamine is a strong base. Triethanolamine can also be abbreviated as TEOA, which can help to distinguish it from triethylamine. It is a colourless compound although samples may appear yellow because of impurities. |
|---|
| Structure | |
|---|
| Synonyms: | - -triethanol
- 2,2', 2''-Nitrilotriethanol
- 2,2',2''-Nitrilotri-Ethanol
- 2,2',2''-Nitrilotriethanol
- 2,2',2''-Nitrilotris(ethanol)
- 2,2',2''-Nitrilotris-Ethanol
- 2,2',2''-Nitrilotris[ethanol]
- 2,2',2''-Trihydroxy-Triethylamine
- 2,2',2-Nitrilotris(ethanol)
- 2,2'2''-Nitrilotris-Ethanol
- Daltogen
- H3tea
- N(CH2CH2OH)3
- Nitrilo-2,2',2"
- Nitrilo-2,2',2''-triethanol
- Nitrilotriethanol
- Salicylate triethanolamine
- Salicylate trolamine
- Salicylic acid triethanolamine
- Salicylic acid trolamine
- Sterolamide
- Sting-Kill
- TEA
- Tea (amino alcohol)
- Teoa
- Thiofaco T-35
- Tri(hydroxyethyl)amine
- Triaethanolamin-NG
- Triethanolamin
- Triethanolamine
- Triethylolamine
- Trihydroxyethylamine
- Trihydroxytriethylamine
- Tris(2-hydroxyethyl)amine
- Tris(b-hydroxyethyl)amine
- Tris(beta-hydroxyethyl)amine
- Tris(β-hydroxyethyl)amine
- Trola
- Trolamine
- Trolamine salicylate
- Trolamine salicylic acid
- {2,2',2''-Nitrilotris[ethanol]}
|
|---|
| Chemical Formula: | C6H15NO3 |
|---|
| Weight: | Average: 149.1882
Monoisotopic: 149.105193351 |
|---|
| InChI Key: | GSEJCLTVZPLZKY-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C6H15NO3/c8-4-1-7(2-5-9)3-6-10/h8-10H,1-6H2 |
|---|
| CAS number: | 102-71-6 |
|---|
| IUPAC Name: | 2-[bis(2-hydroxyethyl)amino]ethan-1-ol |
|---|
| Traditional IUPAC Name: | triethanolamine |
|---|
| SMILES: | OCCN(CCO)CCO |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as 1,2-aminoalcohols. These are organic compounds containing an alkyl chain with an amine group bound to the C1 atom and an alcohol group bound to the C2 atom. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic nitrogen compounds |
|---|
| Class | Organonitrogen compounds |
|---|
| Sub Class | Amines |
|---|
| Direct Parent | 1,2-aminoalcohols |
|---|
| Alternative Parents | |
|---|
| Substituents | - Tertiary aliphatic amine
- Tertiary amine
- 1,2-aminoalcohol
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Alcohol
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Liquid |
|---|
| Charge: | 1 |
|---|
| Melting point: | 20.5 °C |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| | Concentration | Strain | Media | Growth Status | Growth System | Temperature | Details |
|---|
| 61± 0 uM | BW25113 | 48 mM Na2HPO4, 22 mM KH2PO4, 10 mM NaCl, 45 mM (NH4)2SO4, supplemented with 1 mM MgSO4, 1 mg/l thiamine·HCl, 5.6 mg/l CaCl2, 8 mg/l FeCl3, 1 mg/l MnCl2·4H2O, 1.7 mg/l ZnCl2, 0.43 mg/l CuCl2·2H2O, 0.6 mg/l CoCl2·2H2O and 0.6 mg/l Na2MoO4·2H2O. 4 g/L Gluco | Stationary Phase, glucose limited | Bioreactor, pH controlled, O2 and CO2 controlled, dilution rate: 0.2/h | 37 oC | PMID: 17379776 |
|
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (3 TMS) | splash10-03di-1970000000-1cdc1ba11e5a386f1352 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-014i-9800000000-98de38ceda39aaf45b22 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - GC-MS (Non-derivatized) | splash10-03di-1970000000-1cdc1ba11e5a386f1352 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-015j-8900000000-5fc1298b8ec2e7f10e7c | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (3 TMS) - 70eV, Positive | splash10-0ir0-6391000000-838d443b02ca677fd1e6 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0udi-0900000000-0c50692a60886d3a9842 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0ue9-3900000000-2610bf9e1d6b422b2581 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-00di-9100000000-ad4771662a2213681b1c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-00dl-9000000000-3099cf0c685e5aa6736f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-006w-9000000000-d387d0ee743aefe8efec | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QTOF , positive | splash10-0ue9-4900000000-a63f8f03a9f4f6fe4fd5 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-006w-9000000000-91c4f38d178bece0a215 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 40V, Positive | splash10-0005-9000000000-1ec559c373553cf9e682 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0006-9000000000-d753303481ced16e9306 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-0udi-0900000000-1261e0cc2f3543cd4145 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-1900000000-f63e326d732966e5f5cc | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-0udi-0900000000-8775afda0458474bd80c | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-0050f6f0702985876af3 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-006w-9000000000-2f6d85672905dba735bd | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 20V, Positive | splash10-00dr-9200000000-5d9dbc3748ba56547377 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-2900000000-b8d1e629bb99e801b6c3 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-0udi-0900000000-ca2aa12d755f3b370137 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-1900000000-2b587564dc84bdc0bc33 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-01pa-9300000000-57766f4027077ecafb7e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-0002-0900000000-b274020e58b6ef8d982c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-0002-1900000000-c82995f1eed61c2b1729 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0fdo-9300000000-db20818734acbebe5241 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-001i-0900000000-67c8bd04b17ea1ae5dcf | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0ue9-1900000000-3fdb5cb1f4f838e493be | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0002-9100000000-497aac774da51dfe8d7b | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-066r-9400000000-4742c6b283ff3c63f5cf | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Ishii, N., Nakahigashi, K., Baba, T., Robert, M., Soga, T., Kanai, A., Hirasawa, T., Naba, M., Hirai, K., Hoque, A., Ho, P. Y., Kakazu, Y., Sugawara, K., Igarashi, S., Harada, S., Masuda, T., Sugiyama, N., Togashi, T., Hasegawa, M., Takai, Y., Yugi, K., Arakawa, K., Iwata, N., Toya, Y., Nakayama, Y., Nishioka, T., Shimizu, K., Mori, H., Tomita, M. (2007). "Multiple high-throughput analyses monitor the response of E. coli to perturbations." Science 316:593-597. Pubmed: 17379776
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|