| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-07-30 14:55:31 -0600 |
|---|
| Update Date | 2015-06-03 17:21:16 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | Thiomethylgalactoside |
|---|
| Description | Thiomethylgalactoside belongs to the class of Hexoses. These are monosaccharides in which the sugar unit is a hexose. (inferred from compound structure) |
|---|
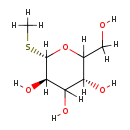
| Structure | |
|---|
| Synonyms: | - (3R,5R,6S)-2-(hydroxymethyl)-6-methylsulfanyloxane-3,4,5-triol
- (3R,5R,6S)-2-(Hydroxymethyl)-6-methylsulphanyloxane-3,4,5-triol
|
|---|
| Chemical Formula: | C7H14O5S |
|---|
| Weight: | Average: 210.248
Monoisotopic: 210.056194248 |
|---|
| InChI Key: | LZFNFLTVAMOOPJ-XMAYFYEJSA-N |
|---|
| InChI: | InChI=1S/C7H14O5S/c1-13-7-6(11)5(10)4(9)3(2-8)12-7/h3-11H,2H2,1H3/t3?,4-,5?,6+,7-/m0/s1 |
|---|
| CAS number: | 155-30-6 |
|---|
| IUPAC Name: | (3R,5R,6S)-2-(hydroxymethyl)-6-(methylsulfanyl)oxane-3,4,5-triol |
|---|
| Traditional IUPAC Name: | (3R,5R,6S)-2-(hydroxymethyl)-6-(methylsulfanyl)oxane-3,4,5-triol |
|---|
| SMILES: | [H]OC([H])([H])C1([H])O[C@@]([H])(SC([H])([H])[H])[C@]([H])(O[H])C([H])(O[H])[C@@]1([H])O[H] |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as thioglycosides. These are glycoside in which a sugar group is bonded through one carbon to another group via a S-glycosidic bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Thioglycosides |
|---|
| Alternative Parents | |
|---|
| Substituents | - S-glycosyl compound
- Oxane
- Monosaccharide
- Monothioacetal
- Secondary alcohol
- Oxacycle
- Organoheterocyclic compound
- Sulfenyl compound
- Polyol
- Hydrocarbon derivative
- Primary alcohol
- Organosulfur compound
- Alcohol
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 92102 | | Kegg ID | Not Available | | ChemSpider ID | 83151 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|