| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-07-30 14:55:14 -0600 |
|---|
| Update Date | 2015-06-03 17:21:05 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
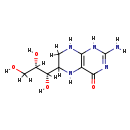
| Name: | Tetrahydromonapterin |
|---|
| Description | Tetrahydromonapterin is a member of the chemical class known as Biopterins and Derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. Tetrahydromonapterin is a major pterin in Escherichia coli and is hypothesized to be the cofactor for phenylalanine hydroxylase (PhhA) in Pseudomonas aeruginosa, but neither its biosynthetic origin nor its cofactor role has been clearly demonstrated. Collectively, these data establish that tetrahydromonapterin formation requires both FolX and FolM, that tetrahydromonapterin is the physiological cofactor for PhhA, and that tetrahydromonapterin can outrank folate as an end product of pterin biosynthesis. (PMID 19897652) |
|---|
| Structure | |
|---|
| Synonyms: | |
|---|
| Chemical Formula: | C9H15N5O4 |
|---|
| Weight: | Average: 257.2465
Monoisotopic: 257.112403993 |
|---|
| InChI Key: | XHIXPVCTDRNTTC-YQVKZWHSSA-N |
|---|
| InChI: | InChI=1S/C9H15N5O4/c10-9-13-7-5(8(18)14-9)12-3(1-11-7)6(17)4(16)2-15/h3-4,6,12,15-17H,1-2H2,(H4,10,11,13,14,18)/t3?,4-,6-/m0/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 2-amino-6-[(1S,2S)-1,2,3-trihydroxypropyl]-1,4,5,6,7,8-hexahydropteridin-4-one |
|---|
| Traditional IUPAC Name: | 2-amino-6-[(1S,2S)-1,2,3-trihydroxypropyl]-5,6,7,8-tetrahydro-1H-pteridin-4-one |
|---|
| SMILES: | [H]OC([H])([H])[C@]([H])(O[H])[C@@]([H])(O[H])C1([H])N([H])C2=C(N([H])C(=NC2=O)N([H])[H])N([H])C1([H])[H] |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as biopterins and derivatives. These are coenzymes containing a 2-amino-pteridine-4-one derivative. They are mainly synthesized in several parts of the body, including the pineal gland. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organoheterocyclic compounds |
|---|
| Class | Pteridines and derivatives |
|---|
| Sub Class | Pterins and derivatives |
|---|
| Direct Parent | Biopterins and derivatives |
|---|
| Alternative Parents | |
|---|
| Substituents | - Biopterin
- Aminopyrimidine
- Pyrimidone
- Secondary aliphatic/aromatic amine
- Pyrimidine
- Heteroaromatic compound
- Vinylogous amide
- 1,3-aminoalcohol
- Secondary alcohol
- 1,2-aminoalcohol
- Azacycle
- Secondary amine
- Polyol
- Hydrocarbon derivative
- Primary amine
- Primary alcohol
- Organopnictogen compound
- Organooxygen compound
- Organonitrogen compound
- Organic oxygen compound
- Organic nitrogen compound
- Amine
- Alcohol
- Organic oxide
- Aromatic heteropolycyclic compound
|
|---|
| Molecular Framework | Aromatic heteropolycyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | 0 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | Tetrahydromonapterin Biosynthesis | PW002043 | 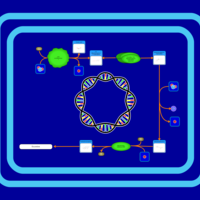   |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- Pribat, A., Blaby, I. K., Lara-Nunez, A., Gregory, J. F. 3rd, de Crecy-Lagard, V., Hanson, A. D. (2010). "FolX and FolM are essential for tetrahydromonapterin synthesis in Escherichia coli and Pseudomonas aeruginosa." J Bacteriol 192:475-482. Pubmed: 19897652
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 71177 | | HMDB ID | Not Available | | Pubchem Compound ID | 45479608 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | CPD0-2101 | | EcoCyc ID | CPD0-2101 |
|
|---|