| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-07-30 14:55:08 -0600 |
|---|
| Update Date | 2015-06-03 17:21:01 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
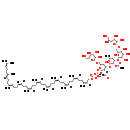
| Name: | O16 antigen undecaprenyl diphosphate |
|---|
| Description | O16 antigen undecaprenyl diphosphate is a member of the chemical class known as Polyprenols. These are prenols with more than 4 consecutive isoprene units. O16 antigen is a repetitive glycan polymer contained within an LPS. It is also called O polysaccharide, or O side-chain of the bacteria. The O antigen is attached to the core oligosaccharide, and comprises the outermost domain of the LPS molecule. The composition of the O chain varies from strain to strain. For example, there are over 160 different O antigen structures produced by different E. coli strains. |
|---|
| Structure | |
|---|
| Synonyms: | - O16 Antigen undecaprenyl diphosphoric acid
|
|---|
| Chemical Formula: | C89H145NO32P2 |
|---|
| Weight: | Average: 1803.0386
Monoisotopic: 1801.922494573 |
|---|
| InChI Key: | NYDKXESIKHJNQU-FIYHCCFRSA-L |
|---|
| InChI: | InChI=1S/C89H147NO32P2/c1-52(2)26-16-27-53(3)28-17-29-54(4)30-18-31-55(5)32-19-33-56(6)34-20-35-57(7)36-21-37-58(8)38-22-39-59(9)40-23-41-60(10)42-24-43-61(11)44-25-45-62(12)46-47-112-123(106,107)122-124(108,109)121-85-70(90-64(14)93)82(74(99)69(116-85)51-110-86-78(103)75(100)72(97)67(49-92)115-86)119-89-84(114-65(15)94)83(71(96)63(13)113-89)120-88-79(104)76(101)73(98)68(117-88)50-111-87-80(105)77(102)81(118-87)66(95)48-91/h26,28,30,32,34,36,38,40,42,44,46,63,66-89,91-92,95-105H,16-25,27,29,31,33,35,37,39,41,43,45,47-51H2,1-15H3,(H,90,93)(H,106,107)(H,108,109)/p-2/b53-28+,54-30+,55-32-,56-34-,57-36-,58-38-,59-40-,60-42-,61-44-,62-46- |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | N-(4-{[3-(acetyloxy)-4-{[6-({[5-(1,2-dihydroxyethyl)-3,4-dihydroxyoxolan-2-yl]oxy}methyl)-3,4,5-trihydroxyoxan-2-yl]oxy}-5-hydroxy-6-methyloxan-2-yl]oxy}-5-hydroxy-2-{[hydroxy({[(2Z,6Z,10Z,14Z,18Z,22Z,26Z,30Z,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl phosphonato]oxy})phosphoryl]oxy}-6-({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-3-yl)ethanecarboximidate |
|---|
| Traditional IUPAC Name: | N-(4-{[3-(acetyloxy)-4-{[6-({[5-(1,2-dihydroxyethyl)-3,4-dihydroxyoxolan-2-yl]oxy}methyl)-3,4,5-trihydroxyoxan-2-yl]oxy}-5-hydroxy-6-methyloxan-2-yl]oxy}-5-hydroxy-2-({hydroxy[(2Z,6Z,10Z,14Z,18Z,22Z,26Z,30Z,34E,38E)-3,7,11,15,19,23,27,31,35,39,43-undecamethyltetratetraconta-2,6,10,14,18,22,26,30,34,38,42-undecaen-1-yl phosphonato]oxyphosphoryl}oxy)-6-({[3,4,5-trihydroxy-6-(hydroxymethyl)oxan-2-yl]oxy}methyl)oxan-3-yl)ethanecarboximidate |
|---|
| SMILES: | [H]\C(CC\C(C)=C(/[H])CC\C(C)=C(\[H])CC\C(C)=C(\[H])CC\C(C)=C(\[H])CC\C(C)=C(\[H])CC\C(C)=C(\[H])CC\C(C)=C(\[H])CC\C(C)=C(\[H])CC\C(C)=C(\[H])COP([O-])(=O)OP(O)(=O)OC1OC(COC2OC(CO)C(O)C(O)C2O)C(O)C(OC2OC(C)C(O)C(OC3OC(COC4OC(C(O)CO)C(O)C4O)C(O)C(O)C3O)C2OC(C)=O)C1N=C(C)[O-])=C(\C)CCC=C(C)C |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as polyprenyl phospho carbohydrates. These are polyprenyl phosphates with a carbohydrate moiety attached to it. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Lipids and lipid-like molecules |
|---|
| Class | Prenol lipids |
|---|
| Sub Class | Polyprenols |
|---|
| Direct Parent | Polyprenyl phospho carbohydrates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Polyterpenoid
- Bactoprenol diphosphate
- Polyprenyl phospho carbohydrate
- Oligosaccharide phosphate
- Polyprenyl monophosphate
- Oligosaccharide
- Polyprenyl phosphate skeleton
- N-acyl-alpha-hexosamine
- O-glycosyl compound
- Glycosyl compound
- Organic pyrophosphate
- Isoprenoid phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Oxane
- Organic phosphoric acid derivative
- Acetamide
- Tetrahydrofuran
- Secondary carboxylic acid amide
- Secondary alcohol
- Carboxylic acid ester
- Carboxamide group
- Oxacycle
- Organoheterocyclic compound
- Polyol
- Monocarboxylic acid or derivatives
- Carboxylic acid derivative
- Acetal
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Primary alcohol
- Organooxygen compound
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Organic anion
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | - Lipopolysaccharide biosynthesis ec00540
|
|---|
| EcoCyc Pathways: | - superpathway of lipopolysaccharide biosynthesis LPSSYN-PWY
|
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | Not Available | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | CPD0-2279 | | EcoCyc ID | CPD0-2279 |
|
|---|