| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-07-30 14:55:07 -0600 |
|---|
| Update Date | 2015-09-13 15:15:31 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | N8-Acetylspermidine |
|---|
| Description | N8-Acetylspermidine is a polyamine. The polyamines, found in virtually all living organisms, are a ubiquitous group of compounds that appear to play a vital role in many cellular processes involving nucleic acids including cell growth and differentiation. The polyamines, found in virtually all living organisms, are a ubiquitous group of compounds that appear to play a vital role in many cellular processes involving nucleic acids including cell growth and differentiation. Acetylation on the terminal nitrogen adjacent to the 4-carbon chain produces N8-acetylspermidine. This reaction is catalyzed by spermidine N8-acetyltransferase and does not result in the conversion of spermidine to putrescine but, instead, the product undergoes deacetylation. |
|---|
| Structure | |
|---|
| Synonyms: | - N-[4-(3-Aminopropylamino)butyl]acetamide
- N-[4-[(3-Aminopropyl)amino]butyl]-Acetamide
- N-{4-[(3-Aminopropyl)amino]butyl}acetamide
- N8-Acetylspermidine
- N8-monoacetylspermidine
|
|---|
| Chemical Formula: | C9H21N3O |
|---|
| Weight: | Average: 187.2825
Monoisotopic: 187.168462309 |
|---|
| InChI Key: | FONIWJIDLJEJTL-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C9H21N3O/c1-9(13)12-8-3-2-6-11-7-4-5-10/h11H,2-8,10H2,1H3,(H,12,13) |
|---|
| CAS number: | 13431-24-8 |
|---|
| IUPAC Name: | N-{4-[(3-aminopropyl)amino]butyl}acetamide |
|---|
| Traditional IUPAC Name: | N8-acetylspermidine |
|---|
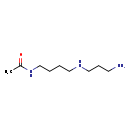
| SMILES: | CC(=O)NCCCCNCCCN |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as carboximidic acids. These are organic acids with the general formula RC(=N)-OH (R=H, organic group). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboximidic acids and derivatives |
|---|
| Sub Class | Carboximidic acids |
|---|
| Direct Parent | Carboximidic acids |
|---|
| Alternative Parents | |
|---|
| Substituents | - Organic 1,3-dipolar compound
- Propargyl-type 1,3-dipolar organic compound
- Secondary amine
- Secondary aliphatic amine
- Carboximidic acid
- Organic nitrogen compound
- Organic oxygen compound
- Organopnictogen compound
- Hydrocarbon derivative
- Primary amine
- Organooxygen compound
- Organonitrogen compound
- Primary aliphatic amine
- Amine
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Solid |
|---|
| Charge: | 2 |
|---|
| Melting point: | 202-203 °C |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | S-adenosyl-L-methionine biosynthesis | PW000837 |    |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-001l-9200000000-7350029bbf7fca576698 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-000i-0900000000-d04fb233b228bfc46b79 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-01w0-0900000000-e68fd1aa92642ae4208d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-03k9-7900000000-287b3decd26c7daeb830 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0089-9100000000-e42a1f8955afadd5f50d | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - LC-ESI-QQ , positive | splash10-0089-9000000000-b18801afe8921946e40f | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-03k9-4900000000-fc04b14a414610552acd | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-009j-1900000000-ec737abcda867c6557b2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004j-6900000000-c57d66a60207b42d5604 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a4l-9100000000-5e4ff8c1235fb203f05f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-1900000000-ed4e22e75d7e4ab076e2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052o-5900000000-f486a2117b1dd060f58e | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-052f-9000000000-dd7c2661d7b6091c7766 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-000i-0900000000-b573184691a2508c0c96 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-052r-6900000000-9ecb44c4262e0df34ea4 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4l-9000000000-078ef81a1628477a273f | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-000i-0900000000-3aacf768b87a62171bf0 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-0229-9600000000-8855f267bbadb8cff170 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-0a59-9100000000-286ce82869d4963e80f9 | View in MoNA |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Abdel-Monem MM, Merdink JL, Theologides A: Urinary excretion of monoacetyl polyamines in patients with non-Hodgkin's lymphoma. Cancer Res. 1982 May;42(5):2097-8. Pubmed: 7066912
- Hrushesky WJ, Merdink J, Abdel-Monem MM: Circadian rhythmicity of polyamine urinary excretion. Cancer Res. 1983 Aug;43(8):3944-7. Pubmed: 6861156
- Inoue H, Fukunaga K, Munemura S, Tsuruta Y: Simultaneous determination of free and N-acetylated polyamines in urine by semimicro high-performance liquid chromatography using 4-(5,6-dimethoxy-2-phthalimidinyl)-2-methoxyphenylsulfonyl chloride as a fluorescent labeling reagent. Anal Biochem. 2005 Apr 15;339(2):191-7. Pubmed: 15797558
- Mudumba S, Menezes A, Fries D, Blankenship J: Differentiation of PC12 cells induced by N8-acetylspermidine and by N8-acetylspermidine deacetylase inhibition. Biochem Pharmacol. 2002 Jun 1;63(11):2011-8. Pubmed: 12093478
- Seiler N, Graham A, Bartholeyns J: Enhanced urinary excretion of N1-acetylspermidine and the presence of tumors. Cancer Res. 1981 Apr;41(4):1572-3. Pubmed: 6897929
|
|---|
| Synthesis Reference: | Tabor, Herbert; Tabor, Celia W.; De Meis, Leopold. Chemical synthesis of N-acetyl-1,4-diaminobutane, N1-acetylspermidine, and N8-acetylspermidine. Methods Enzymol. (1971), 17(Pt. B), 829-33. |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|