| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-07-30 14:55:05 -0600 |
|---|
| Update Date | 2015-06-03 17:20:59 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | L-Alanyl-D-glutamate |
|---|
| Description | L-alanine-D-glutamate is a dipeptide. These are compounds containing an amide derived from two or more amino carboxylic acid molecules (the same or different) by formation of a covalent bond from the carbonyl carbon of one to the nitrogen atom of another. It is a dipeptide that is a component of the bacterial cell wall. It is a substrate of Alanyl-glutamate epimerase which catalyzes the epimerization of L-Ala-D-Glu to L-Ala-L- Glu and has a role in the recycling of the murein peptide, of which L-Ala-D-Glu is a component. This enzyme also able to catalyze the reverse reaction. |
|---|
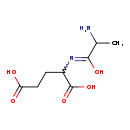
| Structure | |
|---|
| Synonyms: | - L-Ala-γ-D-Glu
- L-Ala-g-D-glu
- L-Ala-gamma-D-Glu
- L-Ala-γ-D-glu
- L-alanine-D-glutamate
- L-Alanine-D-glutamic acid
- L-Alanyl-D-glutamic acid
|
|---|
| Chemical Formula: | C8H13N2O5 |
|---|
| Weight: | Average: 217.1992
Monoisotopic: 217.082446536 |
|---|
| InChI Key: | VYZAGTDAHUIRQA-UHFFFAOYSA-M |
|---|
| InChI: | InChI=1S/C8H14N2O5/c1-4(9)7(13)10-5(8(14)15)2-3-6(11)12/h4-5H,2-3,9H2,1H3,(H,10,13)(H,11,12)(H,14,15)/p-1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | 2-[(2-amino-1-hydroxypropylidene)amino]pentanedioic acid |
|---|
| Traditional IUPAC Name: | 2-[(2-amino-1-hydroxypropylidene)amino]pentanedioic acid |
|---|
| SMILES: | CC(N)C(O)=NC(CCC([O-])=O)C(O)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as dipeptides. These are organic compounds containing a sequence of exactly two alpha-amino acids joined by a peptide bond. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic acids and derivatives |
|---|
| Class | Carboxylic acids and derivatives |
|---|
| Sub Class | Amino acids, peptides, and analogues |
|---|
| Direct Parent | Dipeptides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Alpha-dipeptide
- Glutamic acid or derivatives
- N-acyl-alpha-amino acid
- N-acyl-alpha amino acid or derivatives
- Alpha-amino acid amide
- Alanine or derivatives
- Alpha-amino acid or derivatives
- Dicarboxylic acid or derivatives
- Fatty acid
- Amino acid or derivatives
- Carboxamide group
- Amino acid
- Secondary carboxylic acid amide
- Carboxylic acid
- Organonitrogen compound
- Organic oxygen compound
- Organic oxide
- Primary aliphatic amine
- Organopnictogen compound
- Organic nitrogen compound
- Carbonyl group
- Organooxygen compound
- Primary amine
- Amine
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | Not Available |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -1 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | Not Available |
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | Not Available |
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | Not Available |
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | |
|---|