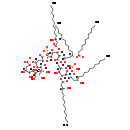| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-07-30 14:55:04 -0600 |
|---|
| Update Date | 2015-06-03 17:20:57 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
| Name: | KDO(2)-lipid IV(A) with laurate |
|---|
| Description | KDO(2)-lipid IV(a) is a saccharolipid. The most familiar saccharolipids are the acylated glucosamine precursors of the lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides of glucosamine, which are derivatized with as many as seven fatty-acyl chains. The minimal lipopolysaccharide required for growth in E. coli is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues. |
|---|
| Structure | |
|---|
| Synonyms: | - kdo(2)-Lipid iv(a) with laate
- kdo(2)-Lipid iv(a) with laic acid
- KDO(2)-lipid IV(A) with lauric acid
- KDO2-(lauroyl)-lipid IVA
|
|---|
| Chemical Formula: | C96H170N2O38P2 |
|---|
| Weight: | Average: 2022.3151
Monoisotopic: 2021.09068211 |
|---|
| InChI Key: | JVUUYJGQIVCMIU-ZODGSCPMSA-H |
|---|
| InChI: | InChI=1S/C96H176N2O38P2/c1-6-11-16-21-26-31-36-41-46-51-66(101)56-76(107)97-81-89(130-79(110)57-67(102)52-47-42-37-32-27-22-17-12-7-2)85(114)74(128-92(81)136-138(122,123)124)64-125-91-82(98-77(108)59-69(54-49-44-39-34-29-24-19-14-9-4)127-78(109)55-50-45-40-35-30-25-20-15-10-5)90(131-80(111)58-68(103)53-48-43-38-33-28-23-18-13-8-3)88(135-137(119,120)121)75(129-91)65-126-95(93(115)116)61-73(84(113)87(133-95)72(106)63-100)132-96(94(117)118)60-70(104)83(112)86(134-96)71(105)62-99/h66-75,81-92,99-106,112-114H,6-65H2,1-5H3,(H,97,107)(H,98,108)(H,115,116)(H,117,118)(H2,119,120,121)(H2,122,123,124)/p-6/t66-,67-,68-,69-,70-,71-,72-,73-,74-,75-,81-,82-,83-,84-,85-,86-,87-,88-,89-,90-,91-,92-,95-,96-/m1/s1 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | (2R,4R,5R,6R)-4-{[(2R,4R,5R,6R)-2-carboxylato-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-2-{[(2R,3S,4R,5R,6R)-5-{[(3R)-3-(dodecanoyloxy)-1-oxidotetradecylidene]amino}-6-{[(2R,3S,4R,5R,6R)-3-hydroxy-5-{[(3R)-3-hydroxy-1-oxidotetradecylidene]amino}-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-3-(phosphonatooxy)oxan-2-yl]methoxy}-5-hydroxyoxane-2-carboxylate |
|---|
| Traditional IUPAC Name: | (2R,4R,5R,6R)-4-{[(2R,4R,5R,6R)-2-carboxylato-6-[(1R)-1,2-dihydroxyethyl]-4,5-dihydroxyoxan-2-yl]oxy}-6-[(1R)-1,2-dihydroxyethyl]-2-{[(2R,3S,4R,5R,6R)-5-{[(3R)-3-(dodecanoyloxy)-1-oxidotetradecylidene]amino}-6-{[(2R,3S,4R,5R,6R)-3-hydroxy-5-{[(3R)-3-hydroxy-1-oxidotetradecylidene]amino}-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-6-(phosphonooxy)oxan-2-yl]methoxy}-4-{[(3R)-3-hydroxytetradecanoyl]oxy}-3-(phosphonatooxy)oxan-2-yl]methoxy}-5-hydroxyoxane-2-carboxylate |
|---|
| SMILES: | [H][C@@](O)(CCCCCCCCCCC)CC(=O)O[C@@]1([H])[C@]([H])(O)[C@@]([H])(CO[C@]2([H])O[C@]([H])(CO[C@@]3(C[C@@]([H])(O[C@@]4(C[C@@]([H])(O)[C@@]([H])(O)[C@]([H])(O4)[C@]([H])(O)CO)C([O-])=O)[C@@]([H])(O)[C@]([H])(O3)[C@]([H])(O)CO)C([O-])=O)[C@@]([H])(OP([O-])([O-])=O)[C@]([H])(OC(=O)C[C@]([H])(O)CCCCCCCCCCC)[C@@]2([H])N=C([O-])C[C@@]([H])(CCCCCCCCCCC)OC(=O)CCCCCCCCCCC)O[C@]([H])(OP(O)(O)=O)[C@]1([H])N=C([O-])C[C@]([H])(O)CCCCCCCCCCC |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as acylaminosugars. These are organic compounds containing a sugar linked to a chain through N-acyl group. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Acylaminosugars |
|---|
| Alternative Parents | |
|---|
| Substituents | - Oligosaccharide phosphate
- Oligosaccharide
- Acylaminosugar
- Saccharolipid
- Hexose phosphate
- Pentacarboxylic acid or derivatives
- N-acyl-alpha-hexosamine
- C-glucuronide
- C-glycosyl compound
- Glycosyl compound
- O-glycosyl compound
- Beta-hydroxy acid
- Ketal
- Fatty acid ester
- Fatty amide
- Fatty acyl
- Hydroxy acid
- N-acyl-amine
- Alkyl phosphate
- Organic phosphoric acid derivative
- Oxane
- Pyran
- Phosphoric acid ester
- Carboxamide group
- Secondary alcohol
- Secondary carboxylic acid amide
- Carboxylic acid ester
- Carboxylic acid derivative
- Organoheterocyclic compound
- Carboxylic acid
- Oxacycle
- Acetal
- Primary alcohol
- Organopnictogen compound
- Alcohol
- Organic oxide
- Carbonyl group
- Hydrocarbon derivative
- Organonitrogen compound
- Organic nitrogen compound
- Organic anion
- Aliphatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aliphatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -5 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Membrane |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | |
|---|
| KEGG Pathways: | - Lipopolysaccharide biosynthesis ec00540
|
|---|
| EcoCyc Pathways: | - superpathway of lipopolysaccharide biosynthesis LPSSYN-PWY
|
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | Not Available |
|---|
| References |
|---|
| References: | - Kanehisa, M., Goto, S., Sato, Y., Furumichi, M., Tanabe, M. (2012). "KEGG for integration and interpretation of large-scale molecular data sets." Nucleic Acids Res 40:D109-D114. Pubmed: 22080510
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
- van der Werf, M. J., Overkamp, K. M., Muilwijk, B., Coulier, L., Hankemeier, T. (2007). "Microbial metabolomics: toward a platform with full metabolome coverage." Anal Biochem 370:17-25. Pubmed: 17765195
- Winder, C. L., Dunn, W. B., Schuler, S., Broadhurst, D., Jarvis, R., Stephens, G. M., Goodacre, R. (2008). "Global metabolic profiling of Escherichia coli cultures: an evaluation of methods for quenching and extraction of intracellular metabolites." Anal Chem 80:2939-2948. Pubmed: 18331064
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | 61524 | | HMDB ID | Not Available | | Pubchem Compound ID | 25244565 | | Kegg ID | Not Available | | ChemSpider ID | 26332260 | | Wikipedia ID | Not Available | | BioCyc ID | Not Available |
|
|---|