| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-07-30 14:54:57 -0600 |
|---|
| Update Date | 2015-09-13 15:15:31 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
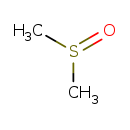
| Name: | Dimethyl sulfoxide |
|---|
| Description | Dimethyl sulfoxide is a highly polar organic liquid, that is used widely as a chemical solvent. Because of its ability to penetrate biological membranes, it is used as a vehicle for topical application of pharmaceuticals. It is also used to protect tissue during cryopreservation. Because of its high boiling point (189 |
|---|
| Structure | |
|---|
| Synonyms: | - (CH3)2SO
- (DMSO)
- (methanesulfinyl)methane
- (methanesulphinyl)methane
- (methylsulfinyl)methane
- (methylsulphinyl)methane
- 1,1'-Sulfinylbis-Methane
- 1,1'-Sulphinylbis-Methane
 N N- Deltan
- Demasorb
- Demavet
- Demeso
- Demsodrox
- Dermasorb
- Diemthyl sulfoxide
- Diemthyl sulphoxide
- Dimethyl sulfoxide
- Dimethyl Sulfoxide (USAN)
- Dimethyl sulfoxide BP
- Dimethyl sulfoxide(USAN)
- Dimethyl sulfoxixde
- Dimethyl sulfur oxide
- Dimethyl sulphoxide
- Dimethyl Sulphoxide (USAN)
- Dimethyl sulphoxide BP
- Dimethyl sulphoxide(USAN)
- Dimethyl sulphoxixde
- Dimethyl sulphur oxide
- Dimethyl sulpoxide
- Dimethyli sulfoxidum
- Dimethyli sulphoxidum
- Dimethylsulfoxid
- Dimethylsulfoxyde
- Dimethylsulphoxid
- Dimethylsulphoxyde
- Dimetil sulfoxido
- Dimetil sulphoxido
- Dimexide
- Dimexidum
- Dipirartril-tropico
- DMSO
- DMSO (methyl sulfoxide)
- DMSO (methyl sulphoxide)
- Dolicur
- Doligur
- Domoso
- Dromisol
- Durasorb
- Gamasol 90
- Herpid
- Hyadur
- Infiltrina
- Kemsol
- Methyl sulfoxide
- Methyl sulphoxide
- Methylsulfinylmethane
- Methylsulphinylmethane
- n
- Rimso 50
- S(O)Me2
- Sclerosol
- Somipront
- Sulfinylbis methane
- Sulfinylbis(methane)
- Sulfinylbis-Methane
- Sulfinylbismethane
- Sulfinyldimethane
- Sulfoxide, dimethyl
- Sulphinylbis methane
- Sulphinylbis(methane)
- Sulphinylbis-Methane
- Sulphinylbismethane
- Sulphinyldimethane
- Sulphoxide, dimethyl
- Syntexan
- Topsym
|
|---|
| Chemical Formula: | C2H6OS |
|---|
| Weight: | Average: 78.133
Monoisotopic: 78.013935504 |
|---|
| InChI Key: | IAZDPXIOMUYVGZ-UHFFFAOYSA-N |
|---|
| InChI: | InChI=1S/C2H6OS/c1-4(2)3/h1-2H3 |
|---|
| CAS number: | 67-68-5 |
|---|
| IUPAC Name: | methanesulfinylmethane |
|---|
| Traditional IUPAC Name: | dimethyl sulfoxide |
|---|
| SMILES: | CS(C)=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as sulfoxides. Sulfoxides are compounds containing a sulfoxide functional group, with the structure RS(=O)R' (R,R' not H). |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organosulfur compounds |
|---|
| Class | Sulfoxides |
|---|
| Sub Class | Not Available |
|---|
| Direct Parent | Sulfoxides |
|---|
| Alternative Parents | |
|---|
| Substituents | - Sulfoxide
- Sulfinyl compound
- Organic oxygen compound
- Organic oxide
- Hydrocarbon derivative
- Aliphatic acyclic compound
|
|---|
| Molecular Framework | Aliphatic acyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Liquid |
|---|
| Charge: | 0 |
|---|
| Melting point: | 18.5 °C |
|---|
| Experimental Properties: | | Property | Value | Source |
|---|
| Water Solubility: | 1000 mg/mL | PhysProp | | LogP: | -1.35; -1.35 [HANSCH,C ET AL. (1995)] | PhysProp |
|
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | dimethyl sulfoxide electron transfer | PW001892 |    |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | - NADH to dimethyl sulfoxide electron transfer PWY0-1348
- formate to dimethyl sulfoxide electron transfer PWY0-1356
|
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | | Spectrum Type | Description | Splash Key | |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03fr-9000000000-f0f118841efd8b3297a3 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03fs-9000000000-945240052686ea267e2b | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03fs-9000000000-378b716bc39417224511 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03fr-9000000000-f0f118841efd8b3297a3 | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03fs-9000000000-945240052686ea267e2b | View in MoNA |
|---|
| GC-MS | GC-MS Spectrum - EI-B (Non-derivatized) | splash10-03fs-9000000000-378b716bc39417224511 | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | splash10-01t9-9000000000-a87469bb1afda1828bbe | View in MoNA |
|---|
| Predicted GC-MS | Predicted GC-MS Spectrum - GC-MS (Non-derivatized) - 70eV, Positive | Not Available | View in JSpectraViewer |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 30V, Positive | splash10-0002-9000000000-b96781268f9bbbcb1544 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 10V, Positive | splash10-004i-9000000000-16f85b2cb3628d8d9358 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 35V, Positive | splash10-03dj-9000000000-c11b31839f52d556db57 | View in MoNA |
|---|
| LC-MS/MS | LC-MS/MS Spectrum - 0V, Positive | splash10-004i-9000000000-6fd68fb341597894c9e9 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-9000000000-c8f7a9f09c8bb456283c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-004i-9000000000-d8d98568ae59afba65e6 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9000000000-e0a36c290f004f302f0b | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-9000000000-89687ba96456a97fb486 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-01t9-9000000000-04ddc1322b21e4817d57 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0a4i-9000000000-d90c2fed7aad139b406c | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Negative | splash10-004i-9000000000-50c596e219854897fad2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Negative | splash10-004i-9000000000-50c596e219854897fad2 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Negative | splash10-0bt9-9000000000-102e2e8c382c8f4c1975 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 10V, Positive | splash10-004i-9000000000-28072bae45639cb50943 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 20V, Positive | splash10-03di-9000000000-670c7176bed65aef23f5 | View in MoNA |
|---|
| Predicted LC-MS/MS | Predicted LC-MS/MS Spectrum - 40V, Positive | splash10-03di-9000000000-9ae0f01cd17306d4ecf0 | View in MoNA |
|---|
| MS | Mass Spectrum (Electron Ionization) | splash10-03fr-9000000000-1db858034b22d1646592 | View in MoNA |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 13C NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
| 1D NMR | 1H NMR Spectrum | Not Available | View in JSpectraViewer |
|---|
|
|---|
| References |
|---|
| References: | - Engelke UF, Tangerman A, Willemsen MA, Moskau D, Loss S, Mudd SH, Wevers RA: Dimethyl sulfone in human cerebrospinal fluid and blood plasma confirmed by one-dimensional (1)H and two-dimensional (1)H-(13)C NMR. NMR Biomed. 2005 Aug;18(5):331-6. Pubmed: 15996001
- Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: | Goeb, Andre. Dimethyl sulfoxide. Ger. Offen. (1976), 12 pp. |
|---|
| Material Safety Data Sheet (MSDS) | Download (PDF) |
|---|
| Links |
|---|
| External Links: | |
|---|