| Record Information |
|---|
| Version | 2.0 |
|---|
| Creation Date | 2012-07-30 14:54:47 -0600 |
|---|
| Update Date | 2015-06-03 17:20:49 -0600 |
|---|
| Secondary Accession Numbers | |
|---|
| Identification |
|---|
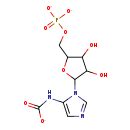
| Name: | 5-Phosphoribosyl-5-carboxyaminoimidazole |
|---|
| Description | 5-carboxyamino-1-(5-phospho-D-ribosyl)imidazole is an intermediate in purine metabolism and IMP biosynthesis via the de novo pathway. It is a substrate of the PurK enzyme which catalyzes the ATP-dependent conversion of 5-aminoimidazole ribonucleotide (AIR) and HCO3- to N5-carboxyaminoimidazole ribonucleotide (N5-CAIR). |
|---|
| Structure | |
|---|
| Synonyms: | - 5-Carboxyamino-1-(5-phospho-D-ribosyl)-imidazole
- 5-phosphoribosyl-5-carboxyaminoimidazole
- N5-CAIR
- N5-CAIR
|
|---|
| Chemical Formula: | C9H11N3O9P |
|---|
| Weight: | Average: 336.1721
Monoisotopic: 336.023290477 |
|---|
| InChI Key: | JHLXDWGVSYMXPL-UHFFFAOYSA-K |
|---|
| InChI: | InChI=1S/C9H14N3O9P/c13-6-4(2-20-22(17,18)19)21-8(7(6)14)12-3-10-1-5(12)11-9(15)16/h1,3-4,6-8,11,13-14H,2H2,(H,15,16)(H2,17,18,19)/p-3 |
|---|
| CAS number: | Not Available |
|---|
| IUPAC Name: | N-(1-{3,4-dihydroxy-5-[(phosphonatooxy)methyl]oxolan-2-yl}-1H-imidazol-5-yl)carbamate |
|---|
| Traditional IUPAC Name: | N-(3-{3,4-dihydroxy-5-[(phosphonatooxy)methyl]oxolan-2-yl}imidazol-4-yl)carbamate |
|---|
| SMILES: | OC1C(O)C(OC1COP([O-])([O-])=O)N1C=NC=C1NC([O-])=O |
|---|
| Chemical Taxonomy |
|---|
| Description | belongs to the class of organic compounds known as pentose phosphates. These are carbohydrate derivatives containing a pentose substituted by one or more phosphate groups. |
|---|
| Kingdom | Organic compounds |
|---|
| Super Class | Organic oxygen compounds |
|---|
| Class | Organooxygen compounds |
|---|
| Sub Class | Carbohydrates and carbohydrate conjugates |
|---|
| Direct Parent | Pentose phosphates |
|---|
| Alternative Parents | |
|---|
| Substituents | - Pentose-5-phosphate
- Pentose phosphate
- Imidazole ribonucleoside
- N-glycosyl compound
- Glycosyl compound
- Monosaccharide phosphate
- Alkyl phosphate
- Phosphoric acid ester
- Organic phosphoric acid derivative
- N-substituted imidazole
- Heteroaromatic compound
- Tetrahydrofuran
- Imidazole
- Azole
- Secondary alcohol
- Carbonic acid derivative
- Carbamic acid derivative
- Carbamic acid
- 1,2-diol
- Oxacycle
- Azacycle
- Organoheterocyclic compound
- Organic nitrogen compound
- Organopnictogen compound
- Organic oxide
- Hydrocarbon derivative
- Organonitrogen compound
- Carbonyl group
- Alcohol
- Organic anion
- Aromatic heteromonocyclic compound
|
|---|
| Molecular Framework | Aromatic heteromonocyclic compounds |
|---|
| External Descriptors | |
|---|
| Physical Properties |
|---|
| State: | Not Available |
|---|
| Charge: | -3 |
|---|
| Melting point: | Not Available |
|---|
| Experimental Properties: | |
|---|
| Predicted Properties | |
|---|
| Biological Properties |
|---|
| Cellular Locations: | Cytoplasm |
|---|
| Reactions: | |
|---|
| SMPDB Pathways: | | purine nucleotides de novo biosynthesis 2 | PW002033 | 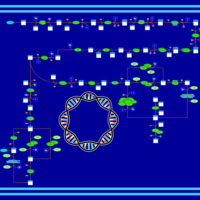 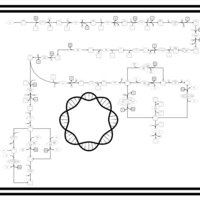 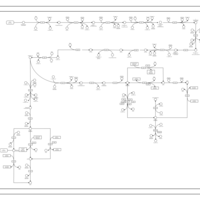 |
|
|---|
| KEGG Pathways: | Not Available |
|---|
| EcoCyc Pathways: | - inosine-5'-phosphate biosynthesis I PWY-6123
|
|---|
| Concentrations |
|---|
| Not Available |
|---|
| Spectra |
|---|
| Spectra: | |
|---|
| References |
|---|
| References: | - Keseler, I. M., Collado-Vides, J., Santos-Zavaleta, A., Peralta-Gil, M., Gama-Castro, S., Muniz-Rascado, L., Bonavides-Martinez, C., Paley, S., Krummenacker, M., Altman, T., Kaipa, P., Spaulding, A., Pacheco, J., Latendresse, M., Fulcher, C., Sarker, M., Shearer, A. G., Mackie, A., Paulsen, I., Gunsalus, R. P., Karp, P. D. (2011). "EcoCyc: a comprehensive database of Escherichia coli biology." Nucleic Acids Res 39:D583-D590. Pubmed: 21097882
|
|---|
| Synthesis Reference: | Not Available |
|---|
| Material Safety Data Sheet (MSDS) | Not Available |
|---|
| Links |
|---|
| External Links: | | Resource | Link |
|---|
| CHEBI ID | Not Available | | HMDB ID | Not Available | | Pubchem Compound ID | 25244287 | | Kegg ID | Not Available | | ChemSpider ID | Not Available | | Wikipedia ID | Not Available | | BioCyc ID | CPD0-181 | | EcoCyc ID | CPD0-181 |
|
|---|